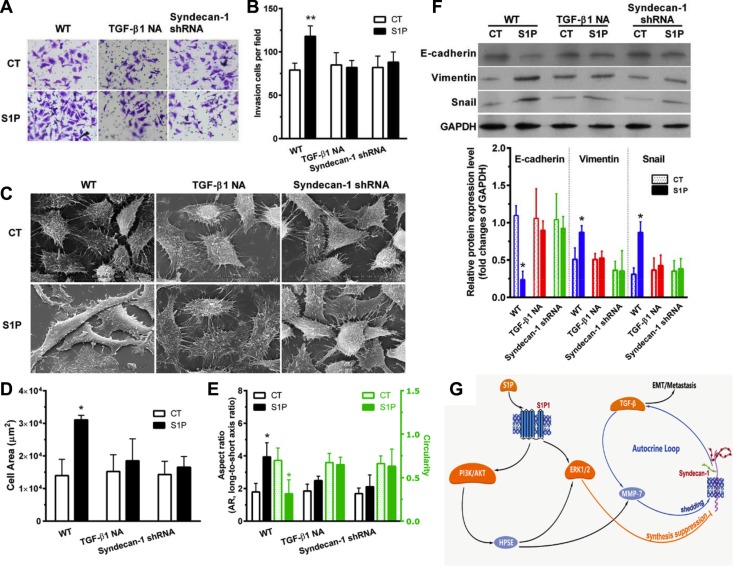
Figure 7. S1P-induced epithelial to mesenchymal transition (EMT) was mediated by syndecan-1 and TGF-β1.
HepG2 control and syndecan-1 shRNA cells were treated with 2 μM S1P for 72 h with or without an addition of 5 μg/ml neutralizing TGF-β1 antibody. (A) and (B), HepG2 cells knocked down for syndecan-1 (syndecan-1 shRNA) compared with control cells were plated to invade through Matrigel-coated Transwell. An addition of 5 μg/ml neutralizing TGF-β1 antibody abolished S1P-induced cell invasion. The invaded cells were quantified and representative images are present. (C), (D), and (E), representative images were photographed under a scanning electron microscope (C). Alterations in cell area (D) and morphology (the aspect ratio and circularity, E) were evaluated by using image J. The results represent the mean ± S.D. of 80 cells from at least three images. (F) expression of epithelial phenotype marker E-cadherin and mesenchymal phenotype marker Vimentin, as well as transcription regulatory protein Snail, were analyzed by Western blotting with densitometric quantification. (G) a schematic summary of S1P-induced EMT via an MMP-7/syndecan-1/TGF-β1 autocrine loop. This pathway involves activation of PI3K/AKT signaling pathways via S1P1, which triggers an enzyme HPSE, leading to an increase in expression and activity of MMP-7 and leading to activation of the ERK1/2 signaling pathway. MMP-7 mediated the shedding of syndecan-1. ERK1/2 mediates the suppression of syndecan-1. The loss of syndecan-1 causes an increase in TGF-β1 secretion and expression. The limited protracted increase in TGF-β1 further enhances MMP-7 activity, leading to syndecan-1 shedding, thus forming an MMP-7/syndecan-1/TGF-β1 autocrine loop. Finally, TGF-β1 and syndecan-1 are essential for S1P-induced epithelial to mesenchymal transition. Syndecan-1 probably behaves as a brake of the autocrine loop and an operator modulated forming of the metastasis-permissive microenvironment. *P < 0.05 as compared with WT control cells (ANOVA).