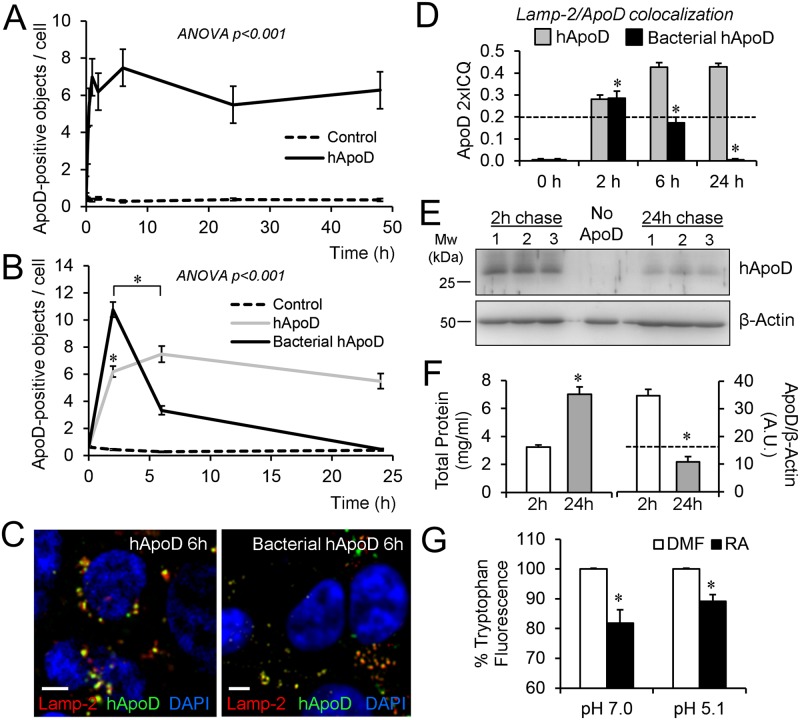
Fig 5. ApoD stability in LELC is glycosylation-dependent and its Lipocalin fold maintains lipid binding functionality in acidic pH.
A. Number of ApoD-positive objects in HEK293T cells after exposure to 500 nM native human ApoD protein (2h pulse/48h chase). B. A 2h pulse/24h chase experiment is performed with bacterially-expressed ApoD protein. Control in A and B are HEK293T cells not exposed to ApoD. C. Colocalization of ApoD with Lamp-2 (6h chase) after exposure to either native human ApoD or bacterially-expressed human ApoD for 2h. D. Lamp-2 colocalization index referenced to ApoD signal (ApoD 2xICQ) in HEK293T in a 2h pulse/24h chase experiment with native or bacterially-expressed ApoD. E. Immunoblot analysis of ApoD loss rate in HEK293T subject to 2h pulse/24h chase with native human ApoD. Equal amounts of total protein (40 μg) are loaded from three independent cultures. A control culture with no ApoD addition is shown in the center lane. F. Total protein concentration duplicates during the experiment due to cell division (left graph). The dashed line marks (right graph) the concentration value expected for ApoD following dilution by cell division. G. Tryptophan fluorescence quenching analysis of retinoic acid (RA) binding to native human ApoD at neutral and acidic pH (pH value selected from peak of lysosomal-pH distribution upon 2h PQ treatment; see Fig 4D). Dimethylformamide (DMF) was used as carrier. Error bars represent SEM in all figures. A-D: n = 20 cells from at least two independent experiments. E-G: n = 3–5 independent experiments analyzed; asterisks represent significant differences (p<0.01) assessed by ANOVA with Holm-Sidak post-hoc method. Calibration bars in C: 5 μm.