Abstract
A hypoxic stress which causes apoptosis of cardiomyocytes is the main problem in the ischemic heart disease. Canstatin, a non-collagenous fragment of type IV collagen α2 chain, is an endogenous anti-angiogenic factor. We have previously reported that canstatin has a cytoprotective effect on cardiomyoblasts. In the present study, we examined the effects of canstatin on hypoxia-induced apoptosis in H9c2 cardiomyoblasts. Cell counting assay was performed to determine a cell viability. Western blotting was performed to detect expression of cleaved casepase-3 and phosphorylation of focal adhesion kinase (FAK) and Akt. Immunocytochemical staining was performed to observe a distribution of αv integrin. Hypoxia (1% O2, 48 h) significantly decreased cell viability and increased cleaved caspase-3 expression. Canstatin (10–250 ng/ml) significantly inhibited these changes in a concentration-dependent manner. Cilengitide (1 μM), an αvβ3 and αvβ5 integrin inhibitor, significantly prevented the protective effects of canstatin on cell viability. Canstatin significantly increased phosphorylation of FAK and Akt under hypoxic condition, which were inhibited by cilengitide. LY294002, an inhibitor of phosphatidylinositol-3 kinase/Akt pathway, suppressed the canstatin-induced Akt phosphorylation and reversed the protective effects of canstatin. It was observed that hypoxia caused a localization of αv integrin to focal adhesion. In summary, we for the first time clarified that canstatin inhibits hypoxia-induced apoptosis via FAK and Akt pathways through activating integrins in H9c2 cardiomyoblasts.
Introduction
Ischemic heart disease such as myocardial infarction is one of the leading causes for death throughout the world [1–3]. A hypoxic stress is the main problem in the ischemic heart disease, which induces apoptosis through the activation of caspase-cascade by a release of cytochrome c from mitochondria to cytoplasm [4,5]. Cell death of matured myocardial cells directly causes a fatal cardiac dysfunction [6–8]. Therefore, the control of the hypoxia-induced apoptosis in cardiomyocytes is thought to be an important therapeutic strategy in the treatment of ischemic heart disease.
Type IV collagen, a major component of the basement membrane, consists of a triple helical structure [9,10]. Canstatin, a non-collagenous fragment, is cleaved from type IV collagen α2 chain, an essential component of basement membrane surrounding cardiomyocytes [11–18]. It is presumed that canstatin exerts anti-angiogenic and anti-tumor effects through binding its receptor, αvβ3 and αvβ5 integrins, in endothelial and tumor cells [11–16]. αvβ3 and αvβ5 integrins are colocalized with human coxsackie-adenovirus receptor on the cardiomyocyte sarcolemma in a dilated cardiomyopathy patient [19]. In situ hybridization revealed that intense expression of αv integrin mRNA was seen in rat cardiomyocytes [20]. In primary adult rat ventricular cardiomyocytes, αv and β1, β3 or β5 integrins are required in periostin-induced cardiomyocyte cell-cycle reentry [21]. It has been reported that cell surface expression of αvβ3 and αvβ5 integrins is increased by hypoxia stimulation in tumor cells [22,23]. β3 integrin prevents oxidative stress-induced apoptosis in HL-1 mouse cardiomyocyte cell line [24]. It has also been reported that αvβ3 and αvβ5 integrins activated the survival signaling pathway through the activation of Akt [25–27] which was responsible for the protection against ischemia-reperfusion injury in mouse cardiomyocytes [28]. In addition, we previously reported that canstatin inhibited isoproterenol-induced apoptosis of differentiated H9c2 cardiomyoblasts [29]. Thus, it is suggested that canstatin might be an endogenous cardioprotective factor in cardiomyocytes. In this study, we tested the hypothesis that canstatin affects hypoxia-induced apoptosis of cardiomyoblasts through the integrins/Akt pathways.
Materials and methods
Reagents and antibodies
Reagent sources were as follows: recombinant mouse collagen alpha-2 (IV) chain, partial (canstatin) (Cusabio Life science, Hubei, China), cilengitide (Adooq Bioscience, Irvine, CA, USA), LY294002 (Wako, Osaka, Japan). Antibodies sources were as follows: phospho-focal adhesion kinase (FAK) (Ser397), total-FAK, αv integrin, vinculin (Santa Cruz Biotechnology, Santa Cruz, CA, USA); phospho-Akt (Ser473), cleaved caspase-3, total-Akt (Cell Signaling Technology, Beverly, MA, USA), total-actin (Sigma-Aldrich, St. Louis, MO, USA)
Hypoxic conditioning
H9c2 cardiomyoblasts obtained from American Type Culture Collection (CRL-1446; Manassas, VA, USA) were cultured in Dulbecco Modified Eagle's Medium (DMEM; Wako, Osaka, Japan) supplemented with 10% fetal bovine serum (FBS; Invitrogen, Carlsbad, CA, USA or HyClone/GE Healthcare, Little Chalfont, UK) and a mixture of 1% penicillin-streptomycin-amphotericin B (Nacalai Tesque, Kyoto, Japan). H9c2 cells at confluence were starved with serum-free DMEM and incubated in a normoxic condition (20% O2, 5% CO2, 37°C) or a hypoxic condition (1% O2, 5% CO2, 37°C) in the presence of varying drugs.
Phase contrast microscopy
H9c2 cardiomyoblasts were grown to confluent and stimulated with canstatin (10–250 ng/ml) or vehicle of canstatin (20 mM Tris, 500 mM L-arginine, 50% glycerol, pH 8.0 for control group) for 48 h. Cell morphology was observed with a phase contrast microscope (CKX-31, OLYMPUS, Tokyo, Japan).
Cell counting assay
Living cell number was counted by a cell counting kit 8 (CC8; Dojindo, Kumamoto, Japan) as described previously [30]. After the stimulation with hypoxia (48 h) in the presence or absence of canstatin (10–250 ng/ml), the cells were washed with Tris-buffered saline (TBS, pH 7.4) and treated with CC8 solution (50 μl/1.0 ml medium) for 1 h at 37°C. The absorbance of the media at 485 nm was measured using a standard microplate reader (Tristar, Berthold Technology, Bad Wildbad, Germany).
Western blotting
Western blotting was performed as described previously [30]. After the stimulation of the cells with hypoxia (24–48 h) in the presence or absence of canstatin (10–250 ng/ml), cilengitide (1 μM) and/or LY294002 (10 μM), total cell lysates were harvested by homogenizing the cells with Triton X-100-based lysis buffer (Cell Signaling Technology) with a protease inhibitor cocktail (Nacalai Tesque). Protein concentration was determined using a bicinchoninic acid method (Pierce, Rockfold, IL, USA). Equal amounts of proteins (10 μg) were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (7.5–14%) and transferred to nitrocellulose membrane (Pall Corporation, Ann Arbor, MI, USA) or polyvinylidene fluoride membrane (ATTO, Tokyo Japan). After being blocked with 0.5% skim milk (for total proteins) or 3% bovine serum albumin (for phosphorylated proteins), the membranes were incubated with primary antibody overnight at 4°C. After incubation with secondary antibody, the chemiluminescence signal was detected by EZ-ECL Western blotting detection reagents (Biological Industries, Kibbutz Beit, Haemek, Israel) using the ATTO light capture system (AE- 6972; ATTO Co., Tokyo, Japan).
Immunocytochemical staining
Immunocytochemical staining was performed as described previously [31]. Twenty four hours after incubation in a normoxic condition (20% O2, 5% CO2, 37°C) or a hypoxic condition (1% O2, 5% CO2, 37°C), the H9c2 cardiomyoblasts were fixed with 4% paraformaldehyde at 4°C for 10 min. Then the cells were permeabilized with 0.2% Triton X-100 (Sigma-Aldrich) at room temperature for 1 min. The permeabilized cells were blocked with 5% normal goat serum for 1 h and incubated with anti-αv integrin and anti-vinculin antibody overnight at 4°C. The cells were incubated with Alexa 488 dye conjugated goat anti-rabbit IgG at room temperature for 1 h. The images were blindly captured using TrueChromeII Plus (BioTool, Gunma, Japan).
Statistical analysis
Data were shown as mean ± standard error of the mean (S.E.M). Statistical evaluations were performed by one-way analysis of variance (ANOVA) followed by Bonferroni's post hoc test. A value of p<0.05 was considered as statistically significant.
Results
Canstatin inhibits hypoxia-induced apoptosis of H9c2 cardiomyoblasts
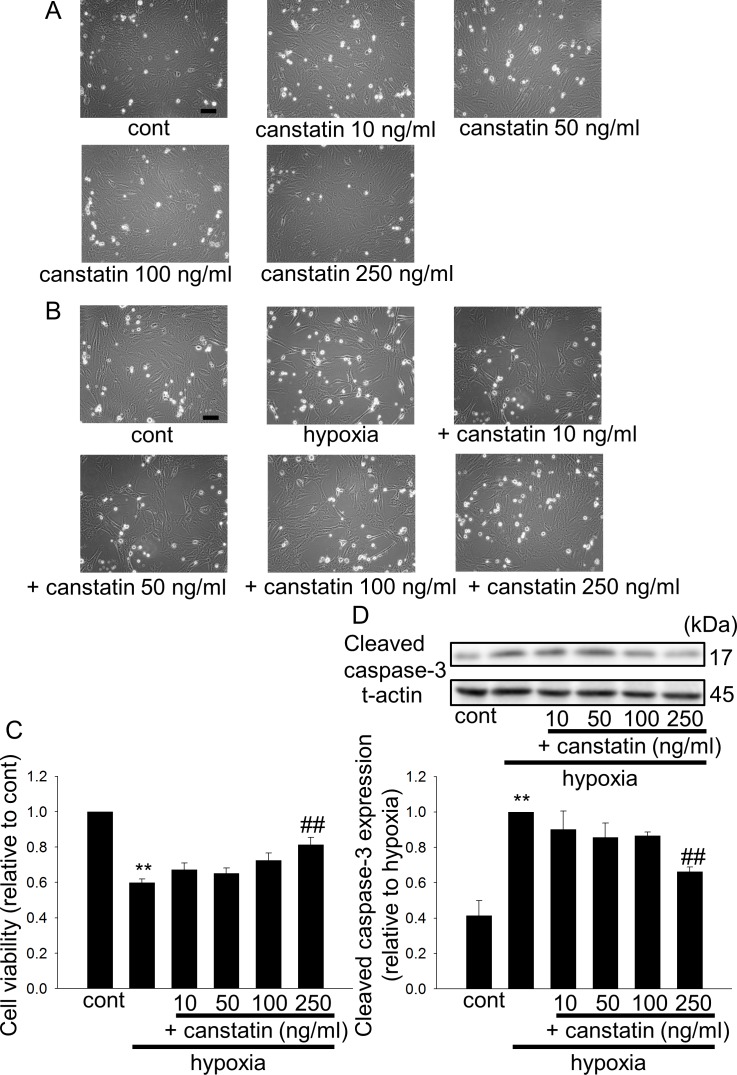
We first confirmed that canstatin-alone treatment (10–250 ng/ml, 48 h) had no effect on the morphology of H9c2 cardiomyoblasts under normoxia (Fig 1A, n = 3). We also confirmed that a higher concentration of canstatin (1 μg/ml) under normoxia had no effect on cell viability (data not shown, n = 6). We next examined the effects of canstatin on hypoxia-induced morphological changes in H9c2 cardiomyoblasts. Morphological changes of cell death, such as detachment of cells, were observed in hypoxia (48 h)-stimulated cells, which were suppressed by canstatin (10–250 ng/ml) in a concentration-dependent manner (n = 7, Fig 1B). We also found that hypoxia (48 h) significantly decreased cell viability (p<0.01 vs. cont), which was significantly suppressed by canstatin in a concentration-dependent manner (10–250 ng/ml) (n = 5, p<0.01 vs. hypoxia, Fig 1C). Canstatin also inhibited the hypoxia (48 h)-induced increases of cleaved caspase-3 expression (p<0.01 vs. cont) in a concentration-dependent manner (10–250 ng/ml) (n = 5, p<0.01 vs. hypoxia, Fig 1D).
Fig 1. Canstatin inhibits hypoxia-induced apoptosis of H9c2 cardiomyoblasts.
(A) H9c2 cardiomyoblasts were treated with canstatin (10–250 ng/ml) for 48 h under normoxia (20% O2, 5% CO2, 37°C). Representative phase-contrast microscopic images of H9c2 cells after vehicle (cont) or canstatin-alone treatment (10–250 ng/ml, 48 h) were shown (n = 3). Scale bar represents 100 μm. (B-D) H9c2 cardiomyoblasts were stimulated with hypoxia for 48 h in the presence or absence of canstatin (10–250 ng/ml). (B) Representative phase-contrast microscopic images of H9c2 cells after treatment with vehicle (cont), hypoxia-alone, or hypoxia + canstatin (250 ng/ml) were shown (n = 7). Scale bar represents 100 μm. (C) After the stimulation with hypoxia, living cell number was counted by a colorimetric method using cell counting kit-8. The normalized cell number relative to cont was shown as mean ± standard error of the mean (S.E.M.). (n = 5). **: p<0.01 vs. cont, ##: p<0.01 vs. hypoxia. (D) After the stimulation with hypoxia, total cell lysates of H9c2 cardiomyoblasts were harvested. Expression of cleaved caspase-3 and total actin (t-actin) was determined by Western blotting. Representative blots of cleaved casepase-3 and t-actin were shown (upper). Levels of cleaved caspase-3 were corrected by t-actin, and the normalized expression relative to hypoxia-alone treatment was shown as mean ± S.E.M. (n = 5). **: p<0.01 vs. cont, ##: p<0.01 vs. hypoxia (lower).
Canstatin suppresses hypoxia-induced apoptosis in H9c2 cardiomyoblasts through activating integrins
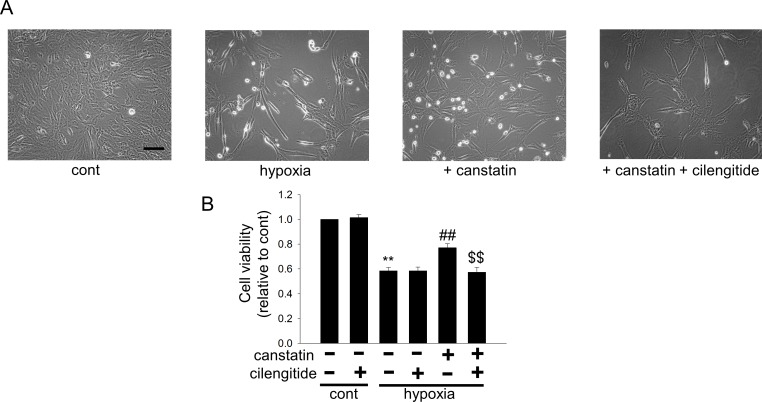
It is presumed that canstatin binds αvβ3 and αvβ5 integrins in endothelial and tumor cells [14,15]. Therefore, we investigated whether canstatin exerts the cytoprotective effects through the interaction with αvβ3 and αvβ5 integrins by using cilengitide (1 μM), an αvβ3 and αvβ5 integrin inhibitor. Canstatin significantly inhibited the hypoxia (48 h)-induced decreases of cell viability, which was significantly suppressed by cilengitide (n = 5, p<0.01 vs. cont, p<0.01 vs. hypoxia, and p<0.01 vs. hypoxia + canstatin, Fig 2A and 2B).
Fig 2. Canstatin suppresses hypoxia-induced apoptosis in H9c2 cardiomyoblasts through the activation of αvβ3 and/or αvβ5 integrins.
H9c2 cardiomyoblasts were stimulated with hypoxia for 48 h in the presence or absence of canstatin (10–250 ng/ml) and cilengitide (1 μM), an αvβ3 and/or αvβ5 integrin inhibitor. (A) Representative phase-contrast microscopic images of H9c2 cells after treatment with vehicle (cont), hypoxia-alone, hypoxia + canstatin (250 ng/ml), or hypoxia + canstatin (250 ng/ml) + cilengitide (1 μM) were shown (n = 5). Scale bar represents 100 μm. (B) After the stimulation with hypoxia for 48 h in the presence or absence of canstatin and cilengitide, living cell number was counted by a colorimetric method using cell counting kit-8. The normalized cell number relative to cont was shown as mean ±S.E.M (n = 5). **: p<0.01 vs. cont, ##: p<0.01 vs. hypoxia, $$: p<0.01 vs. hypoxia + canstatin.
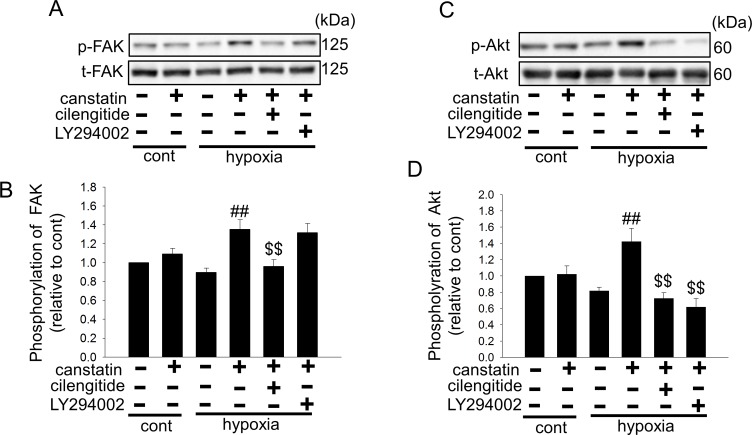
Canstatin enhances phosphorylation of FAK and Akt under hypoxia
αvβ3 and αvβ5 integrins are known to activate Akt and subsequent survival signaling pathway [25–27]. Therefore, we investigated whether canstatin affects the phosphorylation of FAK and Akt. Canstatin-alone treatment (250 ng/ml, 24 h) had no effect on the phosphorylation of FAK and Akt under normoxia (Fig 3A–3D). Interestingly, canstatin (250 ng/ml, 24 h) significantly enhanced phosphorylation of FAK (n = 6, p<0.01 vs. hypoxia) and Akt (n = 4, p<0.01 vs. hypoxia) under hypoxia (Fig 3A–3D). Cilengitide (1 μM) significantly inhibited the canstatin-induced phosphorylation of FAK (n = 6, p<0.01 vs. hypoxia + canstatin) and Akt (n = 4, p<0.01 vs. hypoxia + canstatin) (Fig 3A and 3B). We confirmed that LY29002 (10 μM), a phosphatidylinositol-3 kinase (PI3K)/Akt pathway inhibitor, significantly inhibited the canstatin-induced phosphorylation of Akt (n = 4, p<0.01 vs. hypoxia + canstatin) but not phosphorylation of FAK (Fig 3C and 3D).
Fig 3. Canstatin enhances phosphorylation of focal adhesion kinase (FAK) and Akt under hypoxic condition.
After the stimulation with hypoxia for 24 h in the presence or absence of canstatin (250 ng/ml), cilengitide (1 μM), or LY294002 (10 μM), an inhibitor of phosphatidylinositol-3 kinase (PI3K)/Akt pathway, total cell lysates of H9c2 cardiomyoblasts were harvested. Expression of phospho-FAK (p-FAK) and phospho-Akt (Ser473) (p-Akt) was determined by Western blotting. (A, C) Representative blots of p-FAK and total-FAK (t-FAK) (A) or p-Akt and total-Akt (t-Akt) (C) were shown. (B, D) Levels of phosphorylated proteins were corrected by total proteins, and the normalized expression relative to cont was shown as mean ± S.E.M. (B: FAK: n = 6, D: Akt: n = 4). ##: p<0.01 vs. hypoxia, $$: p<0.01 vs. hypoxia + canstatin.
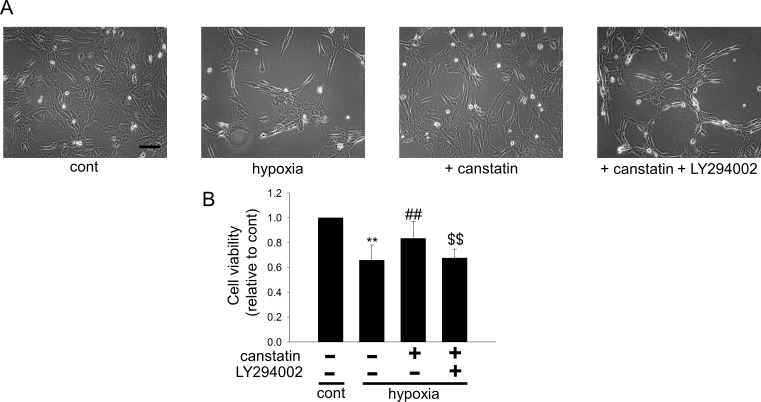
Canstatin inhibits hypoxia-induced apoptosis of H9c2 cardiomyoblasts via PI3K/Akt pathway
We next investigated whether canstatin exerts the cytoprotective effects via PI3K/Akt pathway by using LY294002 (10 μM). Canstatin significantly inhibited the hypoxia (48 h)-induced decreases of cell viability, which was significantly suppressed by LY294002 (n = 6, p<0.01 vs. hypoxia + canstatin, Fig 4A and 4B).
Fig 4. Canstatin inhibits hypoxia-induced apoptosis of H9c2 cardiomyoblasts via PI3K/Akt pathway.
H9c2 cardiomyoblasts were stimulated with hypoxia for 48 h in the presence or absence of canstatin (10–250 ng/ml) and LY294002 (10 μM). (A) Representative phase-contrast microscopic images of H9c2 cells after treatment with vehicle (cont), hypoxia-alone, hypoxia + canstatin (250 ng/ml), or hypoxia + canstatin (250 ng/ml) + LY294002 (10 μM) were shown (n = 4). Scale bar represents 100 μm. (B) After the stimulation with hypoxia for 48 h in the presence or absence of canstatin and LY294002, living cell number was counted by a colorimetric method using cell counting kit-8. The normalized cell number relative to cont was shown as mean ± S.E.M (n = 6). **: p<0.01 vs. cont, ##: p<0.01 vs. hypoxia, $$: p<0.01 vs. hypoxia + canstatin.
Recruitment of αv integrin to the focal adhesion
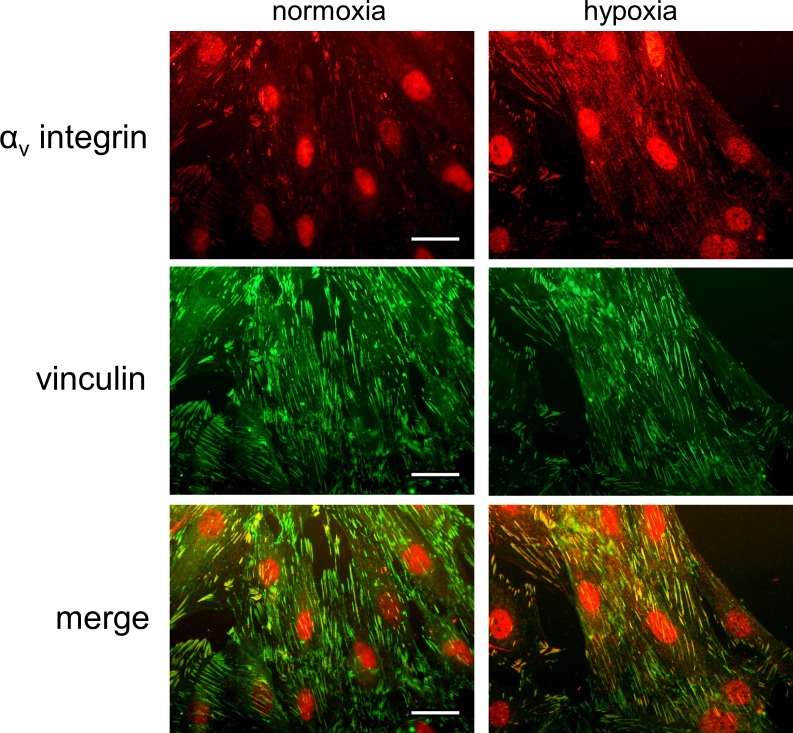
It has been reported that hypoxia induces the recruitment of αvβ3 and αvβ5 integrins to cell membrane in tumor cells [22,23]. We finally investigated whether hypoxia stimulation causes a localization of αv integrin to focal adhesion in H9c2 cardiomyoblasts by performing a double immunofluorescence staining of αv integrin and vinculin, a focal adhesion marker. Co-localization of αv integrin and vinculin was observed prominently in hypoxia (24 h)-stimulated H9c2 cardiomyoblasts compared with normoxia (n = 4, Fig 5). Canstatin and/or cilengitide did not affect the hypoxia-induced recruitment of αv integrin to focal adhesion (data not shown, n = 4).
Fig 5. Hypoxia induces translocation of αv integrin to focal adhesion.
H9c2 cardiomyoblasts were stimulated with normoxia or hypoxia for 24 h. Then a double immunocytochemical staining of αv integrin and vinculin was performed. Representative images of immunofluorescence staining of αv integrin (red), vinculin (green) and merge in H9c2 cells under normoxia (left) or hypoxia (right) were shown (n = 4). A yellow color represents co-localization of αv integrin and vinculin. Scale bar represents 50 μm.
Discussion
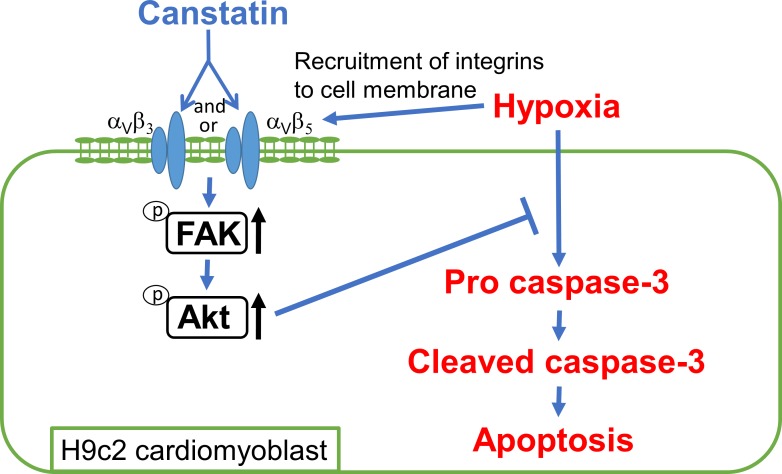
In this study, we for the first time demonstrated that canstatin has a cytoprotective effect on hypoxia-induced apoptosis in H9c2 cardiomyoblasts through the activation of αvβ3 and/or αvβ5 integrins and the phosphorylation of FAK and Akt. (Fig 6).
Fig 6. Schematic model for cytoprotective effects of canstatin on hypoxia-induced apoptosis of H9c2 cardiomyoblasts.
Hypoxia induces the recruitment of integrins to cell membrane and causes apoptosis through the activating caspase-3 cascade. Canstatin inhibits the hypoxia-induced apoptosis through phosphorylation of FAK and Akt by activating αvβ3 and/or αvβ5 integrins in H9c2 cardiomyoblasts.
Canstatin is produced by cleaving type IV collagen α2 chain which is ubiquitously expressed around cardiomyocytes. To the best of our knowledge, the blood concentration of canstatin in a living body has not been clarified. Hamano et al. reported that the blood concentration of tumstatin, an α3 chain cleaved product of type IV collagen, is 336±28 ng/ml in a normal mouse [32]. Thus, we thought that the physiological level of canstatin is ng/ml order and used the concentrations of canstatin ranging from 10 to 250 ng/ml in this study.
Firstly, we confirmed that canstatin-alone treatment had no effect on the morphology of H9c2 cardiomyoblasts. As with the previous report [33], hypoxia significantly increased cleaved caspase-3 expression and caused apoptosis in H9c2 cardiomyoblasts. In this study, canstatin suppressed the hypoxia-induced several apoptotic features, such as morphological damages, decreases of cell viability and increased cleaved caspase-3 expression, in a concentration-dependent manner. We previously reported that canstatin inhibited the isoproterenol-induced apoptosis in differentiated H9c2 cells [29], supporting the present findings that canstatin has a cytoprotective effect in cardiac cells. While canstatin is known to exert a pro-apoptotic effect on endothelial and tumor cells [11–16], it showed the opposite effects on the H9c2 cardiomyoblasts. The reason is speculated that the concentration of canstatin used in this study is much lower than the concentration of canstatin (15–20 μg/ml) reported to stimulate apoptosis [11–13,16]. Kamphaus et al. reported that 1 μg/ml canstatin can inhibit proliferation of human umbilical vein endothelial cells (HUVECs), while it (up to 40 μg/ml) had no significant effect on the proliferation of renal carcinoma cells (786–0), prostate cancer cells (PC-3) or human embryonic kidney cells (HEK 293) [11]. We found that canstatin (1 μg/ml) under normoxia had no effect on cell viability (n = 6). It is thus suggested that lower concentration of canstatin might exert cytoprotective effects through the different mechanisms from endothelial cells.
The receptor for canstatin in endothelial and tumor cells was proposed as αvβ3 and αvβ5 integrins [14,15] and these integrin subtypes are expressed in cardiomyocytes [19–21]. Therefore, we next investigated whether canstatin exerted the cytoprotective effects against hypoxia through integrins and integrin-related signal pathway in H9c2 cardiomyoblasts. Cilengitide, an αvβ3 and αvβ5 integrin inhibitor, significantly suppressed the cytoprotective effect of canstatin. It has also been reported that FAK/Akt pathway, which is involved in cell survival, exists downstream of αvβ3/αvβ5 integrins [25–27]. Therefore, we further investigated whether canstatin activates FAK/Akt signaling. While canstatin-alone treatment had no effect on the phosphorylation of FAK and Akt under a normoxic condition, canstatin significantly enhanced phosphorylation of FAK and Akt under a hypoxic condition. In the present study, the mRNA expression of αv, β3 and β5 integrins was not elevated by hypoxia for 24 h (data not shown, n = 3). It has been reported that hypoxia induced the recruitment of αvβ3 and αvβ5 integrins to cell membrane in tumor cells [22,23]. In this study, we confirmed that hypoxia enhanced the recruitment of αv integrin to focal adhesion which was determined by a co-immunostaining with vinculin, a focal adhesion marker protein. Previous study has shown that the overexpression of αvβ5 integrin induced the phosphorylation of FAK in human dermal myofibroblasts [34]. The activation of integrin/FAK/Akt pathway was also shown to be regulated by conformational change of integrin as well as the quantity and clustering of integrin [35]. The conformational change of integrin has been reported to be induced by inside-out signaling derived from various exogenous stimulations. Hypoxia has also been reported to convert the integrin conformation to active form in myocardial cells [36]. Thus, it is proposed that the recruitment to the focal adhesion and conformational change of integrins may be responsible for the activation of FAK/Akt signaling by canstatin under the hypoxic condition but not under the normoxic condition in H9c2 cardiomyoblasts.
We also showed that cilengitide significantly suppressed the phosphorylation of FAK and Akt. Further, LY294002, an inhibitor of PI3K/Akt pathway, significantly suppressed the cytoprotective effect of canstatin on the hypoxia-induced apoptosis. It has been reported that Akt inactivates the caspase pathway by phosphorylating pro-apoptotic proteins like Bad and inhibiting the release of cytochrome c from mitochondria [37,38]. Collectively, canstatin might inhibit the hypoxia-induced caspase-dependent apoptosis pathway through the activation of FAK/Akt signaling via activating αvβ3 and αvβ5 integrins in H9c2 cardiomyoblasts. The integrin ligands containing Arg-Gly-Asp (RGD) are shown to bind a binding pocket, an extracellular domain of integrin α and β chains [39,40]. Although cilengitide has the RGD motif, recombinant canstatin used in this study does not have it. Kireeva et al. reported that CCN1, a matricellular protein, which does not have RGD sequence, adhered to HUVECs via αvβ3 integrin and the adhesion was inhibited by Arg-Gly-Asp-Ser (RGDS) peptides [41]. They also indicated that the binding of RGDS on αvβ3 integrin exerted conformational changes which caused a masking of other binding sites on the receptor for non-RGD-containing ligands [41]. Thus, cilengitide may change the conformation of αvβ3 and/or αvβ5 integrins by binding and inhibit the activation of canstatin.
Canstatin has been reported to inhibit FAK/PI3K/Akt signaling in HUVECs [16]. In the present study, however, canstatin activated FAK/Akt signaling in H9c2 cardiomyoblasts. Legler et al. reported that a cyclic RGD-peptide, which binds αvβ3 integrin, has biphasic effects (an antagonistic phase at high concentrations and an agonistic phase at low concentrations) [42]. It has also been reported that higher concentration (≥ 20 μM) of cilengitide has an anti-angiogenic, while lower concentration (0.2–20 nM) of cilengitide enhances the growth of tumors by promoting vascular endothelial growth factor-mediated angiogenesis [43]. It is thus suggested that lower concentration of canstatin used in the present study might exert agonistic effects on αvβ3 and αvβ5 integrins. Further studies regarding the effects of higher concentration of canstatin on H9c2 cardiomyoblasts might help to clarify the discrepancy.
In conclusion, we for the first time revealed that canstatin inhibits hypoxia-induced apoptosis via FAK and Akt pathways through activating αvβ3 and/or αvβ5 integrins in H9c2 cardiomyoblasts (Fig 6). Our findings indicate canstatin as a new drug target in ischemic heart disease due to its cardioprotective effects against hypoxic stress-induced apoptosis.
Acknowledgments
We sincerely thank Mr. Masaki Hirakawa for capturing the immunofluorescence images.
Data Availability
All relevant data are within the paper.
Funding Statement
This research was supported by JSPS KAKENHI Grant Number 16K08028 [Grant-in-Aid for Scientific Research (C)] (MO) (URL: http://www.jsps.go.jp/english/e-grants/index.html). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Sanchis-Gomar F, Perez-Quilis C, Leischik R, Lucia A. Epidemiology of coronary heart disease and acute coronary syndrome. Ann Transl Med. 2016; 4: 256 10.21037/atm.2016.06.33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Finegold JA, Asaria P, Francis DP. Mortality from ischaemic heart disease by country, region, and age: statistics from World Health Organisation and United Nations. Int J Cardiol. 2013; 168: 934–945. 10.1016/j.ijcard.2012.10.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation. 2016; 133: e38–360. 10.1161/CIR.0000000000000350 [DOI] [PubMed] [Google Scholar]
- 4.Hu J, Chu Z, Han J, Zhang Q, Zhang D, Dang Y, et al. Phosphorylation-dependent mitochondrial translocation of MAP4 is an early step in hypoxia-induced apoptosis in cardiomyocytes. Cell Death Dis. 2014; 5: e1424 10.1038/cddis.2014.369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Narula J, Pandey P, Arbustini E, Haider N, Narula N, Kolodgie FD, et al. Apoptosis in heart failure: release of cytochrome c from mitochondria and activation of caspase-3 in human cardiomyopathy. Proc Natl Acad Sci U S A. 1999; 96: 8144–8149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moe GW, Marin-Garcia J. Role of cell death in the progression of heart failure. Heart Fail Rev. 2016; 21: 157–167. 10.1007/s10741-016-9532-0 [DOI] [PubMed] [Google Scholar]
- 7.Narula J, Haider N, Virmani R, DiSalvo TG, Kolodgie FD, Hajjar RJ, et al. Apoptosis in myocytes in end-stage heart failure. N Engl J Med. 1996; 335: 1182–1189. 10.1056/NEJM199610173351603 [DOI] [PubMed] [Google Scholar]
- 8.Zhou J, Ahmad F, Parikh S, Hoffman NE, Rajan S, Verma VK, et al. Loss of Adult Cardiac Myocyte GSK-3 Leads to Mitotic Catastrophe Resulting in Fatal Dilated Cardiomyopathy. Circ Res. 2016; 118: 1208–1222. 10.1161/CIRCRESAHA.116.308544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hudson BG, Reeders ST, Tryggvason K. Type IV collagen: structure, gene organization, and role in human diseases. Molecular basis of Goodpasture and Alport syndromes and diffuse leiomyomatosis. J Biol Chem. 1993; 268: 26033–26036. [PubMed] [Google Scholar]
- 10.Kuhn K. Basement membrane (type IV) collagen. Matrix Biol. 1995; 14: 439–445. [DOI] [PubMed] [Google Scholar]
- 11.Kamphaus GD, Colorado PC, Panka DJ, Hopfer H, Ramchandran R, Torre A, et al. Canstatin, a novel matrix-derived inhibitor of angiogenesis and tumor growth. J Biol Chem. 2000; 275: 1209–1215. [DOI] [PubMed] [Google Scholar]
- 12.He GA, Luo JX, Zhang TY, Wang FY, Li RF. Canstatin-N fragment inhibits in vitro endothelial cell proliferation and suppresses in vivo tumor growth. Biochem Biophys Res Commun. 2003; 312: 801–805. 10.1016/j.bbrc.2003.11.003 [DOI] [PubMed] [Google Scholar]
- 13.He GA, Luo JX, Zhang TY, Hu ZS, Wang FY. The C-terminal domain of canstatin suppresses in vivo tumor growth associated with proliferation of endothelial cells. Biochem Biophys Res Commun. 2004; 318: 354–360. 10.1016/j.bbrc.2004.04.038 [DOI] [PubMed] [Google Scholar]
- 14.Magnon C, Galaup A, Mullan B, Rouffiac V, Bouquet C, Bidart JM, et al. Canstatin acts on endothelial and tumor cells via mitochondrial damage initiated through interaction with alphavbeta3 and alphavbeta5 integrins. Cancer Res. 2005; 65: 4353–4361. 10.1158/0008-5472.CAN-04-3536 [DOI] [PubMed] [Google Scholar]
- 15.Mundel TM, Kalluri R. Type IV collagen-derived angiogenesis inhibitors. Microvasc Res. 2007; 74: 85–89. 10.1016/j.mvr.2007.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Panka DJ, Mier JW. Canstatin inhibits Akt activation and induces Fas-dependent apoptosis in endothelial cells. J Biol Chem. 2003; 278: 37632–37636. 10.1074/jbc.M307339200 [DOI] [PubMed] [Google Scholar]
- 17.Watanabe T, Kusachi S, Yamanishi A, Kumashiro H, Nunoyama H, Sano I, et al. Localization of type IV collagen alpha chain in the myocardium of dilated and hypertrophic cardiomyopathy. Jpn Heart J. 1998; 39: 753–762. [DOI] [PubMed] [Google Scholar]
- 18.Yamanishi A, Kusachi S, Nakahama M, Ninomiya Y, Watanabe T, Kumashiro H, et al. Sequential changes in the localization of the type IV collagen alpha chain in the infarct zone: immunohistochemical study of experimental myocardial infarction in the rat. Pathol Res Pract. 1998; 194: 413–422. [DOI] [PubMed] [Google Scholar]
- 19.Noutsias M, Fechner H, de Jonge H, Wang X, Dekkers D, Houtsmuller AB, et al. Human coxsackie-adenovirus receptor is colocalized with integrins alpha(v)beta(3) and alpha(v)beta(5) on the cardiomyocyte sarcolemma and upregulated in dilated cardiomyopathy: implications for cardiotropic viral infections. Circulation. 2001; 104: 275–280. [DOI] [PubMed] [Google Scholar]
- 20.Graf K, Neuss M, Stawowy P, Hsueh WA, Fleck E, Law RE. Angiotensin II and alpha(v)beta(3) integrin expression in rat neonatal cardiac fibroblasts. Hypertension. 2000; 35: 978–984. [DOI] [PubMed] [Google Scholar]
- 21.Kuhn B, del Monte F, Hajjar RJ, Chang YS, Lebeche D, Arab S, et al. Periostin induces proliferation of differentiated cardiomyocytes and promotes cardiac repair. Nat Med. 2007; 13: 962–969. 10.1038/nm1619 [DOI] [PubMed] [Google Scholar]
- 22.Cowden Dahl KD, Robertson SE, Weaver VM, Simon MC. Hypoxia-inducible factor regulates alphavbeta3 integrin cell surface expression. Mol Biol Cell. 2005; 16: 1901–1912. 10.1091/mbc.E04-12-1082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Skuli N, Monferran S, Delmas C, Favre G, Bonnet J, Toulas C, et al. Alphavbeta3/alphavbeta5 integrins-FAK-RhoB: a novel pathway for hypoxia regulation in glioblastoma. Cancer Res. 2009; 69: 3308–3316. 10.1158/0008-5472.CAN-08-2158 [DOI] [PubMed] [Google Scholar]
- 24.Shewchuk LJ, Bryan S, Ulanova M, Khaper N. Integrin beta3 prevents apoptosis of HL-1 cardiomyocytes under conditions of oxidative stress. Can J Physiol Pharmacol. 2010; 88: 324–330. 10.1139/Y09-131 [DOI] [PubMed] [Google Scholar]
- 25.Hwang S, Lee HJ, Kim G, Won KJ, Park YS, Jo I. CCN1 acutely increases nitric oxide production via integrin alphavbeta3-Akt-S6K-phosphorylation of endothelial nitric oxide synthase at the serine 1177 signaling axis. Free Radic Biol Med. 2015; 89: 229–240. 10.1016/j.freeradbiomed.2015.08.005 [DOI] [PubMed] [Google Scholar]
- 26.Riopel M, Stuart W, Wang R. Fibrin improves beta (INS-1) cell function, proliferation and survival through integrin alphavbeta3. Acta Biomater. 2013; 9: 8140–8148. 10.1016/j.actbio.2013.05.035 [DOI] [PubMed] [Google Scholar]
- 27.Lane D, Goncharenko-Khaider N, Rancourt C, Piche A. Ovarian cancer ascites protects from TRAIL-induced cell death through alphavbeta5 integrin-mediated focal adhesion kinase and Akt activation. Oncogene. 2010; 29: 3519–3531. 10.1038/onc.2010.107 [DOI] [PubMed] [Google Scholar]
- 28.Fujio Y, Nguyen T, Wencker D, Kitsis RN, Walsh K. Akt promotes survival of cardiomyocytes in vitro and protects against ischemia-reperfusion injury in mouse heart. Circulation. 2000; 101: 660–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Okada M, Morioka S, Kanazawa H, Yamawaki H. Canstatin inhibits isoproterenol-induced apoptosis through preserving mitochondrial morphology in differentiated H9c2 cardiomyoblasts. Apoptosis. 2016; 21: 887–895. 10.1007/s10495-016-1262-1 [DOI] [PubMed] [Google Scholar]
- 30.Okada M, Oba Y, Yamawaki H. Endostatin stimulates proliferation and migration of adult rat cardiac fibroblasts through PI3K/Akt pathway. Eur J Pharmacol. 2015; 750: 20–26. 10.1016/j.ejphar.2015.01.019 [DOI] [PubMed] [Google Scholar]
- 31.Otani K, Okada M, Yamawaki H. Expression pattern and function of tyrosine receptor kinase B isoforms in rat mesenteric arterial smooth muscle cells. Biochem Biophys Res Commun. 2015; 467: 683–689. 10.1016/j.bbrc.2015.10.084 [DOI] [PubMed] [Google Scholar]
- 32.Hamano Y, Zeisberg M, Sugimoto H, Lively JC, Maeshima Y, Yang C, et al. Physiological levels of tumstatin, a fragment of collagen IV alpha3 chain, are generated by MMP-9 proteolysis and suppress angiogenesis via alphaV beta3 integrin. Cancer Cell. 2003; 3: 589–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu B, Che W, Xue J, Zheng C, Tang K, Zhang J, et al. SIRT4 prevents hypoxia-induced apoptosis in H9c2 cardiomyoblast cells. Cell Physiol Biochem. 2013; 32: 655–62. 10.1159/000354469 [DOI] [PubMed] [Google Scholar]
- 34.Asano Y, Ihn H, Yamane K, Jinnin M, Tamaki K. Increased expression of integrin alphavbeta5 induces the myofibroblastic differentiation of dermal fibroblasts. Am J Pathol. 2006; 168: 499–510. 10.2353/ajpath.2006.041306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hughes PE, Pfaff M. Integrin affinity modulation. Trends Cell Biol. 1998; 8: 359–364. [DOI] [PubMed] [Google Scholar]
- 36.Kalinowski L, Dobrucki LW, Meoli DF, Dione DP, Sadeghi MM, Madri JA, et al. Targeted imaging of hypoxia-induced integrin activation in myocardium early after infarction. J Appl Physiol (1985). 2008; 104: 1504–1512. [DOI] [PubMed] [Google Scholar]
- 37.Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, et al. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997; 91: 231–241. [DOI] [PubMed] [Google Scholar]
- 38.Kennedy SG, Kandel ES, Cross TK, Hay N. Akt/Protein kinase B inhibits cell death by preventing the release of cytochrome c from mitochondria. Mol Cell Biol. 1999; 19: 5800–5810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kapp TG, Rechenmacher F, Neubauer S, Maltsev OV, Cavalcanti-Adam EA, Zarka R, et al. A Comprehensive Evaluation of the Activity and Selectivity Profile of Ligands for RGD-binding Integrins. Sci Rep. 2017; 7: 39805 10.1038/srep39805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mas-Moruno C, Rechenmacher F, Kessler H. Cilengitide: the first anti-angiogenic small molecule drug candidate design, synthesis and clinical evaluation. Anticancer Agents Med Chem. 2010; 10: 753–768. 10.2174/187152010794728639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kireeva ML, Lam SC, Lau LF. Adhesion of human umbilical vein endothelial cells to the immediate-early gene product Cyr61 is mediated through integrin alphavbeta3. J Biol Chem. 1998; 273: 3090–3096. [DOI] [PubMed] [Google Scholar]
- 42.Legler DF, Wiedle G, Ross FP, Imhof BA. Superactivation of integrin alphavbeta3 by low antagonist concentrations. J Cell Sci. 2001; 114: 1545–1553. [DOI] [PubMed] [Google Scholar]
- 43.Reynolds AR, Hart IR, Watson AR, Welti JC, Silva RG, Robinson SD, et al. Stimulation of tumor growth and angiogenesis by low concentrations of RGD-mimetic integrin inhibitors. Nat Med. 2009; 15: 392–400. 10.1038/nm.1941 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.