Abstract
Introduction
Due to an upsurge in antibiotic-resistant infections and lack of therapeutic options, new approaches are needed for treatment. Honey may be one such potential therapeutic option. We investigated the susceptibility of hospital acquired pathogens to four honeys from Wisconsin, United States, and then determined if the antibacterial effect of each honey against these pathogens is primarily due to the high sugar content.
Methods
Thirteen pathogens including: four Clostridium difficile, two Methicillin-resistant Staphylococcus aureus, two Pseudomonas aeruginosa, one Methicillin-Susceptible Staphylococcus aureus, two Vancomycin-resistance Enterococcus, one Enterococcus faecalis and one Klebsiella pneumoniae were exposed to 1-50% (w/v) four Wisconsin honeys and Artificial honey to determine their minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) using the broth dilution method.
Results
Buckwheat honey predominantly exhibited a bactericidal mode of action against the tested pathogens, and this varied with each pathogen. C. difficile isolates were more sensitive to the Wisconsin buckwheat honey as compared to the other pathogens. Artificial honey at 50% (w/v) failed to kill any of the pathogens. The high sugar content of Wisconsin buckwheat honey is not the only factor responsible for its bactericidal activity.
Conclusion
Wisconsin buckwheat honey has the potential to be an important addition to therapeutic armamentarium against resistant pathogens and should be investigated further.
Keywords: Buckwheat honey, susceptibility, antibiotic-resistant, nosocomial pathogen
Introduction
Antibiotic-resistant pathogens (Methicillin-resistant Staphylococcus aureus (MRSA), Pseudomonas aeruginosa, Klebsiella pneumoniae, Clostridium difficile and Enterococcus faecalis) are major causes of severe infections in hospitalized patients leading to longer hospital stays and higher mortality rates worldwide [1, 2]. In the United States, infections associated with antibiotic-resistant organisms occur in over 2 million people and at least 23,000 deaths are recorded annually [3]. According to the WHO report [2], people infected with MRSA are reported to be 64% more likely to die than those infected with antibiotic sensitive strains.
Treatment of antibiotic-resistant associated infection is challenging particularly in healthcare settings due to the increasing trend of antibiotic resistance, side effects of important antibiotics, limited antibiotic options [1, 2] and reduction in new antibiotic discovery endeavors by pharmaceutical companies [2, 4]. Alternative therapeutic interventions that are effective and without adverse effects are urgently needed. Honey is one such promising option.
Natural honey is obtained from nectar collected by honeybees. Its high sugar content coupled with low pH, bee-derived enzymes, bee-derived peptides and phytochemical compounds contribute to its antibacterial action [5–11]. Honey also has antioxidant, anti-inflammatory and anti-hyaluronidase properties which vary depending on the nectar source [5, 12, 13]. The amount of natural phenol in honey plays a significant role in its inhibition activity [12, 13]. Honeys with high concentration of phenol are more likely to possess high inhibitory efficacy than those with low or no phenol.
The use of honey as medicinal remedy was initiated many centuries ago, but recent publications have demonstrated the antibacterial efficacy of honey in in vitro and in vivo [14–22]. The antibacterial mechanism of honey has gradually been unraveled [23]. Evidence from published studies show that honey disrupts cell walls in P. aeruginosa [23] and interrupts cell division in MRSA [24]. Honey also stimulates inflammatory cytokines [25] and has been identified to be a strong scavenger of super peroxide anions and highly effective inhibitor of reactive oxygen species (ROS) stimulated from human polymorphonuclear neutrophils (PMNs) [5]. Both medical grade honey and raw honey have been shown to have broad spectrum antibacterial activity against a plethora of pathogens, including antibiotic-resistant organisms and their biofilms [19, 26, 27]. Furthermore, honey has been shown to heal recalcitrant wounds [28–31]. Effective application of honey promotes wound healing, prevents cross-infection, and repairs tissue [32]. Unlike some antimicrobial agents (such as fluoroquinolones and clindamycin), honey has no record of adverse side effect on tissues [32] or gut [21].
Public interest in the therapeutic use of natural honey in recent times has greatly increased [31]. Licensed medical grade honeys are available in the medical field globally but the majority are derived from Leptospermum species found in Australia and New Zealand. The antibacterial property of medical grade Manuka honey is highly recognized in the research field due to its high unique property, Unique Manuka factor (UMF). Though much work has been done on honey, little is known about the antibacterial efficacy of honey from the United States. In this pilot study, our goals were to compare the efficacies of American honeys and artificial honey on antibiotic-resistant pathogens and then determined if the antibacterial effect of each honey against these pathogensis primarily due to its high sugar content.
Methods
Types of honey
Four Wisconsin honeys (Buckwheat honey, Wild honey, Cranberry honey and Orange blossom honey) and an artificial honey (AH) were studied. Wisconsin honeys were locally produced in Wisconsin, USA, and purchased from a local grocery supermarket. AH (sugar solution) 100 g was comprised of 7.5 g D-(+)-maltose monohydrate, 40.5 g D-(-)-fructose, 1.5 g sucrose, and 33.5 g D-(+)-glucose (Sigma-ALDRICH Co., Missouri, USA) dissolved in 17 ml sterile osmotic reverse water [33]. All honeys were stored at 4°C in a dark environment until use.
Stock solution of honey preparation: A stock honey solution of 50% (w/v), was prepared by dissolving 25 g honey in 50 ml Muller-Hinton broth (MHB) (Thermo Fisher Scientific Remel product, Lenexa, KS, USA) in a 50 ml volumetric flask. The stock solution was diluted for further analysis. Concentrations of honeys were expressed as weight/volume percent (% (w/v)).
Bacteria strains
A total of 13 pathogens (nine aerobic and four anaerobic) were used in the study. The isolates were selected based on their toxigenicity and clinical significance. The pathogens used for the study were received from the archived culture collection of the University of Wisconsin-Madison Infectious Disease Research Laboratory. Their sensitivity to different antibiotics is shown in (Table 1).
Table 1.
Antibiogram characteristics of pathogens
| Pathogens | Antibiotics sensitivity | |
|---|---|---|
| Sensitive to | Resistant to | |
| MSSA ATCC 29213 | Ciprofloxacin, Cefoxitin, Vancomycin | |
| MRSA ATCC 33592 | Cefoxitin | |
| MRSA 0814+ | Cefoxitin | |
| E. feacalis ATCC 51299 | Ciprofloxacin | Vancomycin |
| VRE 002+ | Vancomycin | |
| VRE ATCC 51559+ | Ciprofloxacin, Vancomycin | |
| P. aeruginosa 007+ | Ciprofloxacin | |
| P. aeruginosa ATCC 27853 | Ciprofloxacin | Cefoxitin |
| K. pneumoniae 90 (ESBL)+ | Ciprofloxacin | |
| C. difficile (Ribotype 027) | NT | NT |
| C. difficile (Ribotype 087) | NT | NT |
| C. difficile 0001+ | NT | NT |
| C. difficile 0009+ | NT | NT |
Clinical isolates. ESBL=Extended spectrum beta-lactamase–producing organism. NT= not tested.
The aerobic pathogens consisted of two MRSA (ATTC 33592 and 0814) strains, two P. aeruginosa (ATCC 27853 and ATCC 51559) strains, one Methicillin-Susceptible S. aureus (MSSA) (ATCC 29213) strain, two vancomycin-resistant Enterococcus (VRE) (ATCC 51559 and VRE 002) strains, E. faecalis ATCC 51299, and K. pneumoniae. To ensure viability and purity, each pathogen recovered from a -80°C freezer was sub-cultured on blood agar (BA) plates (Thermo Fisher Scientific Remel product, Lenexa, KS, USA) and incubated aerobically at 37°C for 24 h before susceptibility tests were performed. Overnight cultures were emulsified in phosphate buffered saline (PBS) (Fisher BioReagent, New Jersey, USA) to 0.5 McFarland standard and further diluted in Muller-Hinton Broth-2 (MHB2) (Sigma-ALDRICH Co., Missouri, USA) to approximately 5x106 CFU/ml for immediate testing.
The anaerobic pathogens, four toxigenic C. difficile isolates (ATCC BAA-1870 (Ribotype 027), ATCC 43255 (Ribotype 087), 0001 and 0009), were grown in BBL Brucella Broth (SBB) (Becton Dickinson, Sparks, MD) supplemented with hemin (Sigma-Aldrich CO., St. Louis, MO) and vitamin K (Alfa Aesar, Ward Hill, MA) as recommended by the Clinical and Laboratory Standards Institute (CLSI) document M11-A7 [34]. Incubation was carried out in an anaerobic chamber (Coy Laboratory Products, Inc., Grass Lake, MI) with anaerobic mixed gases (10% H2, 10% CO2, 80% N2). Agar plates and reagents were pre-reduced in an anaerobic cabinet overnight before use. Tested organisms were retrieved from a -80°C storage freezer then sub-cultured at 24 h intervals at least three times on BA plates (Remel, Lenexa, KS, USA) and incubated anaerobically to confirm the purity and viability of the organisms before use. Overnight broth cultures were used for each test.
The overnight cultures, which grew heavily, were diluted in PBS (Fisher BioReagent, New Jersey, USA) to 0.5 McFarland standard and further diluted in SBB to approximately 5x106 CFU/ml before further susceptibility tests were performed. Colony count was also monitored on BA plates to ensure all the wells received equal and accurate amount of inoculum density.
Minimum inhibitory concentration determination
The minimum inhibitory concentration (MIC) was determined in 96 well round-bottomed microtiter plates (Corning Incorporated, New York, USA). Broth microdilution method was used according to CLSI guidelines [34] and [35] with modifications. A volume of 100 µl of varied honey concentrations (0-50%) (w/v) was distributed in wells 1-10. Wells 11 and 12 were considered positive (broth) and negative controls (broth and honey only), respectively. Subsequently, wells 1-11 were seeded with an aliquot of 10 µl of approximately 106 CFU/mL of overnight MHB culture and incubated at 37°C for 24 h aerobically. Positive control and negative control were added to monitor viability and sterility of honey, respectively. Reference stains were also included to monitor consistency. Wells with the lowest honey concentrations which prevented growth/turbidity under a magnifying mirror were considered as MIC. For quality assurance purposes, each experiment was run in triplicate at different occasions. The method was validated by using standard antibiotics against the reference strains and the results compared to that in CLSI literature.
Minimum bactericidal concentration determination
Minimum bactericidal concentration (MBC) was determined by plating 10 µl of content from all the MIC wells without visible growth/turbidity onto antibiotic-free or honey-free BA plates in duplicate and incubating aerobically and anaerobically as required. Positive and negative control wells were included. The lowest honey concentration that killed the organism was considered the MBC. MBCs were determined on three separate occasions.
Statistical analysis
Data was entered into Microsoft Excel® 2010 and analyzed. The Student´s t-test was performed to determine whether the differences in mean of Wisconsin Buckwheat honey (WBH) and the AH for each isolate were significant. A p-value ≤ 0.05 was considered significant. For each honey type, the ratio of MBC to MIC was determined and used to classify the antibacterial activity of the honey as either bactericidal or bacteriostatic. Bactericidal was defined as MBC/MIC ratio less than or equal to 4, while a MBC/MIC ratio above 4 and less than 16 was considered bacteriostatic [36].
Results
The selected pathogens for the study are listed in Table 1. The MICs and MBCs of the selected pathogens are shown on (Figure 1). Generally, WBH exhibited bactericidal mode of action against all the tested organisms with MBC/MIC ≤ 4. WBH exhibited a broad spectrum antimicrobial property but Gram-positive organisms were slightly more susceptible than Gram-negative organisms (Figure 1). There was a dose response relationship between the tested honey and the pathogens. The MICs of the natural honey was very low as compared to that of the AH. For the aerobic organisms, AH inhibited growth at 50% (w/v) whiles that for anaerobic organisms was 40% (w/v). These high concentrations failed to kill any of the organisms. However, WBH demonstrated bactericidal activity with MIC range between 6.25-19% (w/v) and MBC range between (6.25-50% (w/v) (Figure 1). The effect of WBH on VRE 002 and VRE 51559 were high with MBCs of 50 and >50 (% w/v) respectively. There was a significant difference in susceptibility between the WBH and AH for all the tested isolates (p<0.05). The cranberry honey, wild honey and orange blossom honey did not exhibit bactericidal activity at 50% (w/v).
Figure 1.
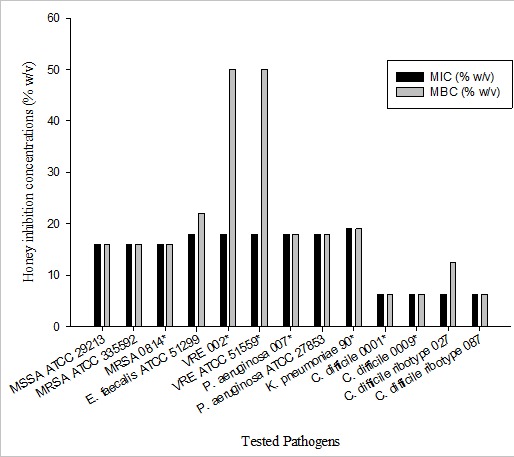
The minimum inhibitory concentrations (MIC) and minimum bactericidal concentrations (MBC) (% w/v) of WBH, on antibiotic-resistant pathogens. The values are represented as mean of triplicate results. The investigation of MIC and MBC values for aerobic and anaerobic isolates were carried out after 24 h and 48 h incubation respectively. American Type Culture Collection (ATCC). +Clinical isolates. ++ MBC >50.
Discussion
The rise in multidrug and extreme antibiotic-resistant pathogens in the healthcare settings is so alarming that it has become necessary to find alternative and effective natural therapeutic agents. In this study, we tested the potency of four local honeys against standardized pathogens (MRSA, P. aeruginosa, MSSA, VRE, E. faecalis, K. pneumoniae and C. difficile). Honeys that did not exhibit bactericidal activity at 50% (w/v) during the study were excluded.
Our study demonstrated that MICs of WBH for the four C. difficile isolates were the same (6.25 % w/v) with MBC ranging between 6.25-12.5% (w/v). Despite the different nectar sources of the honeys, these results were congruent with previous work by Hammond and Donkor [17] who demonstrated that Woundcare Manuka honey inhibited C. difficile. The striking antibacterial activity of WBH on C. difficile is an evidence that some natural honeys have the potential to treat C. difficile infection. These honeys could be developed for oral administration and could represent a big step forward in the treatment of antibiotic-resistant nosocomial infections.
Additionally, WBH exhibited bactericidal effects on MRSA, VRE, E. faecalis, K. pneumoniae, and P. aeruginosa with MIC range 16-20 (% w/v) and MBC range 16-50 (% w/v). A similar study by Brudzynski et al. [37] indicated that Canadian buckwheat honey displayed a powerful bactericidal effect against antibiotic-resistant pathogens. Cooper et al [38] also established that the effect of Medihoney on P. aeruginosa ATCC 27853 was bactericidal with MIC of 15.7% (w/v). In comparison, some of our results varied from those obtained by other investigators. Factors responsible for these discrepancies may include change in honey activity level from batch to batch, and materials, methods, and techniques employed. These findings add up to previous results obtained on susceptibility of multiple organisms to honey of different nectar sources and geographical origin [15, 16, 19, 22, 33, 37, 39].
To investigate the role of high sugar content of selected honeys on antibacterial activity, we exposed test pathogens to various concentrations of AH (10-50% w/v) to mimic the main sugar composition in natural honeys. Our findings indicated that a high AH concentration (50% w/v) failed to completely prevent any of the pathogens from growth, whereas WBH exhibited bactericidal activity at very low honey concentrations (6.25% w/v). A similar conclusion was reported by [16]. Likewise at 50% (w/v), cranberry honey, wild honey and orange blossom honey supported the growth of all the studied pathogens. This further confirms that bactericidal activity of honey is not solely due to the presence of high sugar content and that varying potent antibacterial compounds in honey may work synergistically to extensively disrupt cells and lysis of pathogens as reported by Henriques et al. [23]. This study suggests that WBH may have a complex composition that could effectively resist multiple antibiotic-resistant pathogens to thrive.
WBH remarkably displayed broad spectrum antimicrobial activity in this study. Other published research have demonstrated that phenolic compound in buckwheat honey is high and this influences its antibacterial property [5, 12, 13]. Based on this, we can deduce that the phenol content in Wisconsin honey may be high, and hence could be a major factor contributing to its antibacterial activity against these important hospital acquired pathogens. The potent bactericidal effect of WBH suggests that more honeys need to be tested to supplement existing medical grade honeys and standard antibiotics to effectively control the increasing antibiotic-resistant pathogens during therapy. If processed, WBH could be employed therapeutically. Furthermore, buckwheat honey is common in the United States and may be cost effective than imported medical grade Manuka honey but further study is required.
Our findings suggest that it is important to carry out further investigations to determine the rate and concentrations at which WBH inhibits pathogens. Investigating the effect of WBH on C. difficile spores could be advantageous since C. difficile infections mode of transmission is also through spores which can survive for a long time in the contaminated environment. Since microbial biofilm delays wound healing, it is important to determine if WBH prevents or disrupts biofilm formed by pathogens in the study that could possibly cause wound infections. Furthermore, it will be essential to determine if the antibacterial efficacy of the selected buckwheat honey is representative of all buckwheat honeys in the whole state of Wisconsin or the United States.
Conclusion
We provide the first data on antibacterial efficacy of buckwheat honey from Wisconsin, USA against nosocomial or hospital acquired pathogens. Our data demonstrate that antibiotic and multiple drug resistant pathogen(s) were susceptible to WBH. We also deduced that the antibacterial effect of WBH on pathogens including C. difficile was not mainly due to its high sugar content. Future work should include in vivo studies to examine efficacy and mechanism of action.
What is known about this topic
Antibiotic resistant pathogens is a major cause of deaths in hospitals;
Antibacterial activity of honey from different nectar sources differ;
High sugar content in honey is not solely responsible for antibacterial activity of honey.
What this study adds
Wisconsin buckwheat honey has bactericidal activity against antibiotic-resistant pathogens;
This is the first data on Wisconsin buckwheat honey against antibiotic-resistant pathogens including Clostridium difficile;
Wisconsin buckwheat honey has the potential to treat nosocomial associated infections.
Acknowledgments
We thank Dr Benjamin Darien (University of Wisconsin School of Veterinary Medicine), Sara Fleming (University of Wisconsin School of Medicine and Public Health) and Dr Eric Sampane Donkor (University of Cambridge, UK) for the suggestions they provided during the study. This work is supported by a Patient Safety Center from the VA National Center for Patient Safety. Dr Safdar is supported by a VA MERIT award and by an R03 from the Agency for Healthcare Research and Quality.
Competing interests
The authors declare no competing interests.
Authors’ contributions
ENH and NS conceived and designed the experiment. The experiment was carried out by ENH and interpretation of the data was done by ENH, JSM and MD. The manuscript was written and reviewed by ENH, MD, JSM and NS. NS contributed reagents/materials/analysis tools. All authors have read and agreed to the final manuscript.
References
- 1.Safdar N. Clostridium difficile: The Emerging Epidemic. Mayo Clin Proc. 2012 Nov;87(11):1037–9. doi: 10.1016/j.mayocp.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. WHO’s first global report on antibiotic resistance reveals serious, worldwide threat to public health. http://www.who.int/mediacentre/news/releases/2014/amr-report/en/. Accessed 29 June 2015. [Google Scholar]
- 3.The Centers for Disease Control and Prevention. Antibiotic Resistance Threats in the United States. 2013. http://www.cdc.gov/drugresistance/threat-report-2013/. Accessed 29 June 2015. [Google Scholar]
- 4.Spellberg B, Guidos R, Gilbert D, Bradley J, Boucher HW, Scheld WM, et al. The epidemic of antibiotic-resistant infections: a call to action for the medical community from the Infectious Diseases Society of America. Clin Infect Dis. 2008 Jan 15;46(2):155–64. doi: 10.1086/524891. [DOI] [PubMed] [Google Scholar]
- 5.van den Berg AJJ, van den Worm E, van Ufford HCQ, Halkes SBA, Hoekstra MJ, Beukelman CJ. An in vitro examination of the antioxidant and anti-inflammatory properties of buckwheat honey. J Wound Care. 2008 Apr;17(4):172–4. 176–8. doi: 10.12968/jowc.2008.17.4.28839. [DOI] [PubMed] [Google Scholar]
- 6.Kato Y, Umeda N, Maeda A, Matsumoto D, Kitamoto N, Kikuzaki H. Identification of a novel glycoside, leptosin, as a chemical marker of manuka honey. J Agric Food Chem. 2012 Apr 4;60(13):3418–23. doi: 10.1021/jf300068w. [DOI] [PubMed] [Google Scholar]
- 7.Kwakman PHS, Velde AA te, de Boer L, Vandenbroucke-Grauls CMJE, Zaat SAJ. Two Major Medicinal Honeys Have Different Mechanisms of Bactericidal Activity. PLoS One. 2011 Mar 4;6(3):e17709. doi: 10.1371/journal.pone.0017709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mavric E, Wittmann S, Barth G, Henle T. Identification and quantification of methylglyoxal as the dominant antibacterial constituent of Manuka (Leptospermum scoparium) honeys from New Zealand. Mol Nutr Food Res. 2008 Apr;52(4):483–9. doi: 10.1002/mnfr.200700282. [DOI] [PubMed] [Google Scholar]
- 9.Molan P. The Antibacterial Activity of Honey 1 the Nature of the Antibacterial Activity. Bee World. 1992;73(1):5–28. [Google Scholar]
- 10.AL-Waili N, Al Ghamdi A, Ansari MJ, Al-Attal Y, Al-Mubarak A, Salom K. Differences in Composition of Honey Samples and Their Impact on the Antimicrobial Activities against Drug Multiresistant Bacteria and Pathogenic Fungi. Arch Med Res. 2013 May;44(4):307–16. doi: 10.1016/j.arcmed.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 11.Brudzynski K, Miotto D. The recognition of high molecular weight melanoidins as the main components responsible for radical-scavenging capacity of unheated and heat-treated Canadian honeys. Food Chem. 2011 Mar;125(2):570–5. doi: 10.1016/j.foodchem.2011.01.075. [DOI] [PubMed] [Google Scholar]
- 12.Kolayli S, Can Z, Yildiz O, Sahin H, Karaoglu SA. A comparative study of the antihyaluronidase, antiurease, antioxidant, antimicrobial and physicochemical properties of different unifloral degrees of chestnut (Castanea sativa Mill) honeys. J Enzyme Inhib Med Chem. 2016 Jul;20:1–9. doi: 10.1080/14756366.2016.1209494. [DOI] [PubMed] [Google Scholar]
- 13.Kolayli S, Sahin H, Can Z, Yildiz O, Sahin K. Honey shows potent inhibitory activity against the bovine testes hyaluronidase. J Enzyme Inhib Med Chem. 2016 Aug;31(4):599–602. doi: 10.3109/14756366.2015.1054819. [DOI] [PubMed] [Google Scholar]
- 14.Abdullah B, Lazim NM, Salim R. The effectiveness of Tualang honey in reducing post-tonsillectomy pain. Kulak Burun Bogaz Ihtis Derg. 2015;25(3):137–43. doi: 10.5606/kbbihtisas.2015.00008. [DOI] [PubMed] [Google Scholar]
- 15.Boateng J, Diunase KN. Comparing the Antibacterial and Functional Properties of Cameroonian and Manuka honeys for potential wound healing-have we come full cycle in dealing with antibiotic resistance? Molecules. 2015 Sep;20(9):16068–84. doi: 10.3390/molecules200916068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carnwath R, Graham EM, Reynolds K, Pollock PJ. The antimicrobial activity of honey against common equine wound bacterial isolates. Vet J. 2014 Jan;199(1):110–4. doi: 10.1016/j.tvjl.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 17.Hammond EN, Donkor ES. Antibacterial effect of Manuka honey on Clostridium difficile. BMC Res Notes. 2013 May;6:188. doi: 10.1186/1756-0500-6-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hawley P, Hovan A, McGahan CE, Saunders D. A randomized placebo-controlled trial of manuka honey for radiation-induced oral mucositis. Support Care Cancer Off J Multinatl Assoc. 2014 Mar;22(3):751–61. doi: 10.1007/s00520-013-2031-0. [DOI] [PubMed] [Google Scholar]
- 19.Kuś PM, Szweda P, Jerković I, Tuberoso CIG. Activity of Polish unifloral honeys against pathogenic bacteria and its correlation with colour, phenolic content, antioxidant capacity and other parameters. Lett Appl Microbiol. 2016 Mar;62(3):269–76. doi: 10.1111/lam.12541. [DOI] [PubMed] [Google Scholar]
- 20.Siavash M, Shokri S, Haghighi S, Shahtalebi MA, Farajzadehgan Z. The efficacy of topical royal jelly on healing of diabetic foot ulcers: a double-blind placebo-controlled clinical trial. Int Wound J. 2015 Apr;12(2):137–42. doi: 10.1111/iwj.12063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wallace A, Eady S, Miles M, Martin H, McLachlan A, Rodier M, et al. Demonstrating the safety of manuka honey UMF 20+ in a human clinical trial with healthy individuals. Br J Nutr. 2010 Apr;103(7):1023–8. doi: 10.1017/S0007114509992777. [DOI] [PubMed] [Google Scholar]
- 22.Wilkinson JM, Cavanagh HMA. Antibacterial activity of 13 honeys against Escherichia coli and Pseudomonas aeruginosa. J Med Food. 2005 Apr;8(1):100–3. doi: 10.1089/jmf.2005.8.100. [DOI] [PubMed] [Google Scholar]
- 23.Henriques AF, Jenkins RE, Burton NF, Cooper RA. The effect of manuka honey on the structure of Pseudomonas aeruginosa. Eur J Clin Microbiol Infect Dis. 2011 Feb;30(2):167–71. doi: 10.1007/s10096-010-1065-1. [DOI] [PubMed] [Google Scholar]
- 24.Jenkins R, Burton N, Cooper R. Manuka honey inhibits cell division in methicillin-resistant Staphylococcus aureus. J Antimicrob Chemother. 2011 Nov;66(11):2536–42. doi: 10.1093/jac/dkr340. [DOI] [PubMed] [Google Scholar]
- 25.Majtan J, Kumar P, Majtan T, Walls AF, Klaudiny J. Effect of honey and its major royal jelly protein 1 on cytokine and MMP-9 mRNA transcripts in human keratinocytes. Exp Dermatol. 2010 Aug;19(8):e73–9. doi: 10.1111/j.1600-0625.2009.00994.x. [DOI] [PubMed] [Google Scholar]
- 26.Al-Nahari AAM, Almasaudi SB, Abd El-Ghany ESM, Barbour E, Al Jaouni SK, Harakeh S. Antimicrobial activities of Saudi honey against Pseudomonas aeruginosa. Saudi J Biol Sci. 2015 Sep;22(5):521–5. doi: 10.1016/j.sjbs.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hammond EN, Donkor ES, Brown CA. Biofilm formation of Clostridium difficile and susceptibility to Manuka honey. BMC Complement Altern Med. 2014 Sep;14:329. doi: 10.1186/1472-6882-14-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cooper RA, Molan PC, Krishnamoorthy L, Harding KG. Manuka honey used to heal a recalcitrant surgical wound. Eur J Clin Microbiol Infect Dis. 2001 Oct;20(10):758–9. doi: 10.1007/s100960100590. [DOI] [PubMed] [Google Scholar]
- 29.Visavadia BG, Honeysett J, Danford M. Manuka honey dressing: an effective treatment for chronic wound infections. Br J Oral Maxillofac Surg. 2008 Dec;46(8):696–7. doi: 10.1016/j.bjoms.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 30.Henatsch D, Wesseling F, Briedé JJ, Stokroos RJ. Treatment of chronically infected open mastoid cavities with medical honey: a randomized controlled trial. Otol Neurotol. 2015 Jun;36(5):782–7. doi: 10.1097/MAO.0000000000000728. [DOI] [PubMed] [Google Scholar]
- 31.Surahio AR, Khan AA, Farooq M, Fatima I. Role of honey in wound dressing in diabetic foot ulcer. J Ayub Med Coll Abbottabad. 2014 Jul-Sep;26(3):304–6. [PubMed] [Google Scholar]
- 32.Molan PC. Potential of Honey in the Treatment of Wounds and Burns. Am J Clin Dermatol. 2001;2(1):13–9. doi: 10.2165/00128071-200102010-00003. [DOI] [PubMed] [Google Scholar]
- 33.Cooper RA, Molan PC, Harding KG. The sensitivity to honey of Gram-positive cocci of clinical significance isolated from wounds. J Appl Microbiol. 2002;93(5):857–63. doi: 10.1046/j.1365-2672.2002.01761.x. [DOI] [PubMed] [Google Scholar]
- 34.Clinical and Laboratory Standards Institute. Clinical and Laboratory Standards Institute: Methods for Antimicrobial Susceptibility Testing of Anaerobic Bacteria; Approved Standard-Seventh Edn. 2. Vol. 27. Wayne, PA: Clinical and Laboratory Standards Institute (CLSI), CLSI document M11-A7; 2007. [Google Scholar]
- 35.Clinical and Laboratory Standards Institute. Clinical and Laboratory Standards Institute: Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard-Ninth Edn. 2. Vol. 32. Wayne, PA: Clinical and Laboratory Standards Institute (CLSI), CLSI document M07-A9; 2012. [Google Scholar]
- 36.May J, Shannon K, King A, French G. Glycopeptide tolerance in Staphylococcus aureus. J Antimicrob Chemother. 1998 Aug;42(2):189–97. doi: 10.1093/jac/42.2.189. [DOI] [PubMed] [Google Scholar]
- 37.Brudzynski K, Abubaker K, Wang T. Powerful bacterial killing by buckwheat honeys is concentration-dependent, involves complete DNA degradation and requires hydrogen peroxide. Front Microbiol. 2012 Jul 4;3:242. doi: 10.3389/fmicb.2012.00242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cooper RA, Jenkins L, Henriques AFM, Duggan RS, Burton NF. Absence of bacterial resistance to medical-grade manuka honey. Eur J Clin Microbiol Infect Dis. 2010 Oct;29(10):1237–41. doi: 10.1007/s10096-010-0992-1. [DOI] [PubMed] [Google Scholar]
- 39.Huttunen S, Riihinen K, Kauhanen J, Tikkanen-Kaukanen C. Antimicrobial activity of different Finnish monofloral honeys against human pathogenic bacteria. APMIS. 2013 Sep;121(9):827–34. doi: 10.1111/apm.12039. [DOI] [PMC free article] [PubMed] [Google Scholar]


