Abstract
Termite mounds built by representatives of the family Termitidae are among the most spectacular constructions in the animal kingdom, reaching 6–8 m in height and housing millions of individuals. Although functional aspects of these structures are well studied, their evolutionary origins remain poorly understood. Australian representatives of the termitid subfamily Nasutitermitinae display a wide variety of nesting habits, making them an ideal group for investigating the evolution of mound building. Because they feed on a variety of substrates, they also provide an opportunity to illuminate the evolution of termite diets. Here, we investigate the evolution of termitid mound building and diet, through a comprehensive molecular phylogenetic analysis of Australian Nasutitermitinae. Molecular dating analysis indicates that the subfamily has colonized Australia on three occasions over the past approximately 20 Myr. Ancestral-state reconstruction showed that mound building arose on multiple occasions and from diverse ancestral nesting habits, including arboreal and wood or soil nesting. Grass feeding appears to have evolved from wood feeding via ancestors that fed on both wood and leaf litter. Our results underscore the adaptability of termites to ancient environmental change, and provide novel examples of parallel evolution of extended phenotypes.
Keywords: parallel evolution, environmental change, extended phenotype
1. Introduction
Parallel evolution is the independent appearance of similar derived phenotypes among closely related species. It has key implications for the debate over the predictability of evolution. On one side of this debate, evolution is proposed to be stochastic and unpredictable [1], while on the other, it is thought to be subject to constraints that limit available phenotypic outcomes [2]. Although parallel genotypic evolution has been shown at lower taxonomic levels, the occurrence of parallel evolution across genera within a clade has only rarely been shown [3]. The potential for climate change to drive parallel evolution also remains understudied.
Aridification across the Australian landmass over the past 20 Myr provided conditions conducive to parallel evolution, as related species in shrinking mesic areas adapted to drier habitats. One prominent feature of the arid Australian landscape is its vast numbers of termite mounds. These fortresses can reach heights of 6–8 m in the case of mounds built by the northern Australian species Nasutitermes triodiae [4]. The functions of mounds are well studied, and include protection from predators, food storage and the regulation of environmental conditions [5]. However, their evolutionary origins remain poorly understood.
Australian members of the Termitidae subfamily Nasutitermitinae (hereafter ‘nasutes’) provide an ideal opportunity to study the origins of mound building, and to investigate potential parallel evolution of this trait. Of 44 described species, 13 species construct mounds [4]; mound building is rare among representatives of the subfamily from other geographical regions. Other species in the subfamily create their nests arboreally (on the outside of trees), within dead wood or within soil [6,7] (figure 1). Australian nasutes are ecologically dominant in savannah habitats and are known for their diverse feeding habits, ranging from sound wood through to rotting wood, leaf litter, grass, soil or a combination of these substrates [4]. This diversity of substrates provides an additional opportunity to investigate potential parallel evolution of diet in these species.
Figure 1.
Phylogenetic tree estimated in a Bayesian analysis of 1800 bp from mitochondrial 12S, 16S, and COX2 genes. Circles at nodes indicate posterior probabilities. Branch lengths are proportional to time, with the scale bar given in millions of years before present. Each of the three Australian lineages is indicated as L1–L3. Branch shading indicates the inferred geographical locations. Example mound and arboreal nests from selected species (a–f) are shown (photo credits: (a) Jan Šobotník, (b) Graham Chapman, (e) Neil Ross, (f) Aaron Stewart). (Online version in colour.)
Here, we perform the first comprehensive investigation of the evolution of mound building and grass feeding in Termitidae, via a phylogenetic analysis of Australian nasutes. We sought to answer the following specific questions: (i) How many times have these traits evolved in this group, and from what ancestral states? (ii) When did mound building and grass feeding evolve, and did their appearance coincide with known geological and climatic events? Our overarching aim was to investigate the potential role of environmental change in driving parallel evolution of these key termite traits.
2. Material and methods
Eighty-six samples of Australian Nasutitermitinae were examined in this study, representing the diversity of the subfamily (32 of the 44 described Australian species, plus another 10 morphospecies not diagnosable to described species). Three mitochondrial genes (COX2, 16S and 12S) and one nuclear locus (ITS1) were sequenced in these taxa, and phylogenetic analyses were carried out on these and other Nasutitermitinae taxa from around the world using maximum likelihood and Bayesian inference. Details of collection locations, identification, DNA extraction, primers and amplification conditions, phylogenetic methods, fossil calibrations and ancestral-state reconstruction methods are provided in the electronic supplementary material.
3. Results and discussion
(a). Colonization of Australia by Nasutitermitinae over the past approximately 20 Ma
Our phylogenetic analyses of mitochondrial genes revealed three well-supported and divergent lineages (L1–L3) of endemic Australian Nasutitermitinae (figure 1). L1 is most closely related to species from Papua New Guinea and South America, which together form a sister clade to L2. L3 is divergent from L1 and L2, and is the sister lineage of a clade containing taxa from Asia. Trees estimated using maximum likelihood and Bayesian inference (figure 1 and electronic supplementary material, figure S2) were generally concordant. Relationships among taxa inferred from ITS1 were generally in agreement with those inferred from mitochondrial sequences (see the electronic supplementary material, figure S3), although we did find evidence of divergent ITS1 copies in some taxa. The overall concordance between the mitochondrial and ITS1 trees confirms previous results [6,8,9] showing that mitochondrial genes are reliable markers for inferring relationships among these taxa.
Our results indicate that Nasutitermitinae colonized Australia on at least two, and probably three, occasions over the past approximately 20 Myr. L3 appears to represent the earliest colonization of Australia, approximately 17.8 Ma (95% credibility interval 12.77–23.53 Ma; figure 1), whereas L1 arrived approximately 12 Ma (95% CI 8.84–17.48 Ma). The timing of colonization of Australia by the ancestors of L2, which is represented by a single species, is unclear. The geographical origins of colonizations by L1–L3 are unclear based on the topology in figure 1. There was no evidence for movement of taxa from Australia to any other regions following their arrival.
(b). Evolution of nesting types
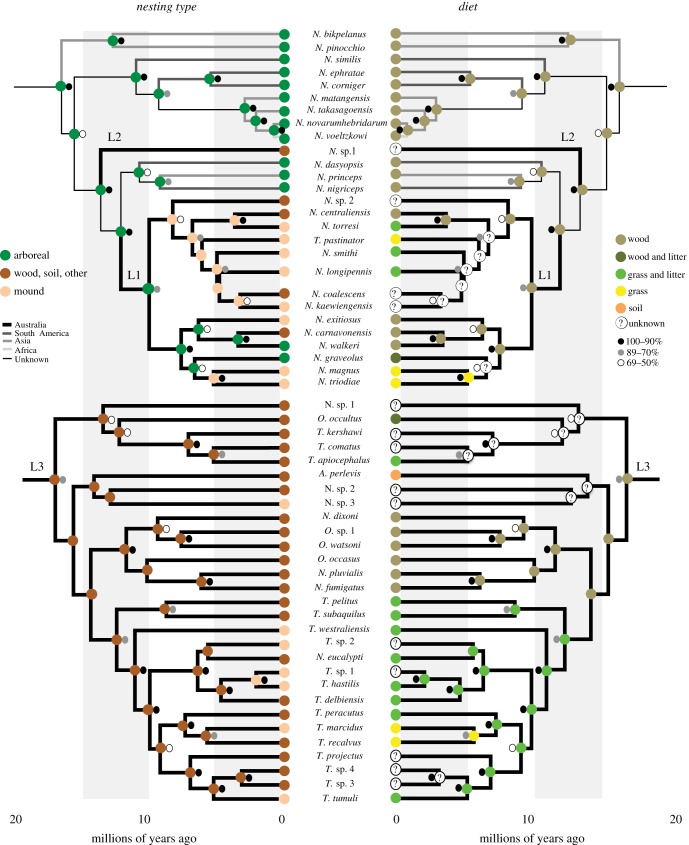
Ancestral-state reconstruction showed that the ancestors of L1 and L2 were arboreal nesters (figure 2 and electronic supplementary material, figure S4). Extant nasute arboreal nesters from Asia, Central and South America, and Africa often have distributions that include coastal areas, and this was presumably the case for their last common ancestor. The proximity of ancestral arboreal nesters to coastal habitats is likely to have facilitated the dispersal of L1 and L2 across oceanic barriers to Australia. Following the establishment of the arboreal L1 ancestor in Australia, mound building was inferred to have evolved on three separate occasions in parallel as this lineage diversified. Nasutitermes walkeri and N. graveolus maintained the ancestral arboreal nesting habitat; like their relatives in other parts of the world, the distributions of these two taxa include coastal areas. In L3, the wood or subterranean nesting habit is present in most extant lineages and was inferred as the ancestral state. Mound building was inferred to have evolved six times in parallel within this lineage (figure 2 and electronic supplementary material, figure S4).
Figure 2.
Reconstruction of ancestral nesting habits and diet for the clades containing Australian lineages L1–L3. The tree is based on the Bayesian estimate of the mitochondrial tree (figure 1); support values (shown) were generally high within each of these lineages. The tree was pruned so that only one sample of each putative species is represented. N., unclassified Nasutitermitinae. The ancestral dietary state of wood feeding in L3 is based on the wider analysis shown in the electronic supplementary material, figure S5. (Online version in colour.)
Mound-building in L1 was inferred to have first evolved approximately 7.5 Ma (95% CI 5.39–10.90 Ma) in the ancestors of N. smithi, N. sp 2 and Tumulitermes pastinator, and approximately 5 Ma (95% CI 4.12–8.35 Ma) in the ancestor of N. triodiae and N. magnus. These inferred times overlap with the hypothesized expansion of open habitats such as sclerophyllous woodlands and savannahs in Australia at around 6–8 Ma [10]. The onset of drier conditions may have spurred the transition from arboreal nesting to the construction of mounds on the soil surface, where moisture could be more easily sourced and retained. Termite mounds typically extend well below the ground, where conditions are more cool and moist [4,5]. The conversion of woodland to savannah habitats is likely to have resulted in fewer trees available for the construction of arboreal nests, and selection for nest construction on the ground. In the case of L3, the timeframe for the evolution of mound building is more difficult to ascertain, but it appears to have occurred within the past approximately 5–6 Ma in three out of six instances (ancestors of T. hastilis + T. sp. 1, T. marcidus and T. tumuli).
In addition to the expansion of open habitats at around 6–8 Ma, Australia experienced prolonged periods of cool and dry conditions during the Pleistocene [10]. The increasing scarcity during these periods of wooden logs, in or under which ancestral taxa from L3 nested, may have driven the construction of mounds in some lineages. A number of L3 taxa (e.g. N. pluvialis, N. dixoni) are distributed in relatively mesic habitats, which may explain why their ancestors did not evolve mound building. The distributions of a number of non-mound building L3 species in arid areas (e.g. T. recalvus, T. peracutus) indicates that such conditions do not necessarily result in the evolution of mound building from wood or soil nesting.
(c). Evolution of diet
The ancestors of each of the three major Australian nasute lineages were inferred to have been wood feeders (electronic supplementary material, figure S5). Within L1, N. graveolus evolved the ability to feed on litter and wood. There were at least two independent transitions from wood feeding to either grass + litter or grass feeding, but the unknown feeding status of some species in L1 makes it difficult to infer ancestral states (figure 2). Within L3, a key transition from wood feeding to grass + leaf-litter feeding occurred early during the evolution of the group, and was followed by a subsequent transition to grass feeding in the ancestors of T. recalvus and T. marcidus (figure 2). In other parts of L3, wood feeding was retained; a lack of information on the feeding habits of the remaining members of L3 precludes the reliable inference of some ancestral states.
During the evolution of grass feeding, ancestral wood feeders might have first evolved the ability to feed on wood and litter. These taxa would then have lost the ability to feed on wood as they transitioned to feeding on litter and grass. Some lineages subsequently transitioned to a strict grass diet. The evolution of diverse feeding habits across L1–L3 may have been influenced by environmental change. The onset of extended periods of arid conditions from 15 Ma onwards [10] caused a reduction in rotten, moist wood, which may have driven the transition to alternative substrates such as leaf litter and grass. Consistent with this, the transition from wood feeding to grass and litter feeding in L3 was estimated to have occurred approximately 12.5 Ma (95% CI 8.84–17.12 Ma; figure 2). The parallel transition to strict grass feeding in the ancestors of N. triodiae/N. magnus and T. marcidus/T. recalvus was inferred to have occurred more recently at 5–6 Ma (95% CI 3.14–8.56 Ma). This timeframe is consistent with the spread of grassland habitats in subtropical savannahs and central Australia in the Late Miocene/Early Pliocene [11]. Further evidence will be needed to test this hypothesis, including additional information on the feeding habits of extant species and ancestral lineages.
4. Conclusion
Our results provide a novel example of parallel evolution across genera in the Nasutitermitinae, the timing of which is consistent with periods of ancient climate change. This group is one of the most ecologically successful groups of termites in Australia [4]. We have shown that its capacity to disperse over oceans, and to repeatedly evolve the ability to build mounds and feed on novel substrates in the face of significant environmental change, appears to have been important in promoting this success. These results emphasize the predictability of evolution under certain ecological conditions. Further studies at the genomic level of Australian nasute termites may shed light on the underlying genetic mechanisms of dietary evolution and the complex phenomenon of mound building.
Supplementary Material
Supplementary Material
Data accessibility
Sequence data have been uploaded to GenBank (accession numbers KX011613-KX011851 and KX977324-KX977386) [12].
Authors' contributions
D.A.A. and A.N. generated sequence data; T.A.E., S.L.C., N.L., D.K.Y. and D.A.A. collected and provided specimens; D.A.A, A.N., N.L. and S.Y.W.H. performed data analysis; D.A.A., T.A.E., S.L.C., D.K.Y., S.Y.W.H. and N.L. interpreted data; N.L., D.A.A., T.A.E. and S.L.C. conceived of the study; N.L. and D.A.A. wrote the manuscript and all authors contributed to the final version and agreed to be accountable for all aspects of the work.
Competing interests
We have no competing interests.
Funding
D.A.A. was supported by a Kuwait Ministry of Higher Education Masters of Science Scholarship. This research was supported by the Commonwealth Environmental Research Facilities Emerging Priorities Program and the Atlas of Living Australia.
References
- 1.Gould SJ. 1989. Wonderful life: the burgess shale and the nature of history. New York, NY: W. W. Norton & Co. [Google Scholar]
- 2.Conway Morris S. 2003. Life's solution: inevitable humans in a lonely universe. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 3.Stern DL. 2013. The genetic causes of convergent evolution. Nat. Rev. Genet. 14, 751–764. ( 10.1038/nrg3483) [DOI] [PubMed] [Google Scholar]
- 4.Gay FJ, Calaby JH. 1970. Termites of the Australian region. In Biology of termites (eds Krishna K, Weesner FM), pp. 393–448. New York, NY: Academic Press. [Google Scholar]
- 5.Korb J. 2010. Termite mound architecture, from function to construction. In Biology of termites: a modern synthesis (eds Bignell DE, Roisin Y, Lo N), pp. 349–374. Dordrecht, The Netherlands: Springer. [Google Scholar]
- 6.Inward DJ, Vogler AP, Eggleton P. 2007. A comprehensive phylogenetic analysis of termites (Isoptera) illuminates key aspects of their evolutionary biology. Mol. Phylogenet. Evol. 44, 953–967. ( 10.1016/j.ympev.2007.05.014) [DOI] [PubMed] [Google Scholar]
- 7.Miller LR. 1997. Systematics of the Australian Nasutitermitinae with reference to evolution within the Termitidae (Isoptera). PhD thesis, Australian National University.
- 8.Miura T, Roisin Y, Matsumoto T. 2000. Molecular phylogeny and biogeography of the nasute termite genus Nasutitermes (Isoptera: Termitidae) in the Pacific tropics. Mol. Phylogenet. Evol. 17, 1–10. ( 10.1006/mpev.2000.0790) [DOI] [PubMed] [Google Scholar]
- 9.Bourguignon T, et al. 2015. The evolutionary history of termites as inferred from 66 mitochondrial genomes. Mol. Biol. Evol. 32, 406–421. ( 10.1093/molbev/msu308) [DOI] [PubMed] [Google Scholar]
- 10.Byrne M, et al. 2011. Decline of a biome: evolution, contraction, fragmentation, extinction and invasion of the Australian mesic zone biota. J. Biogeogr. 38, 1635–1656. ( 10.1111/j.1365-2699.2011.02535.x) [DOI] [Google Scholar]
- 11.Jones RN. 1997. The biogeography of the grasses and lowland grasslands of south-eastern Australia. Adv. Nat. Conserv. 2, 11–18. [Google Scholar]
- 12.Arab D, Namyatova A, Evans TA, Cameron SL, Yeates DK, Ho SYW, Lo N. 2016. Data from: Parallel evolution of mound-building and grass-feeding in Australian nasute termites. GenBank Accession numbers KX011613-KX011851 and KX977324-KX977386. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Arab D, Namyatova A, Evans TA, Cameron SL, Yeates DK, Ho SYW, Lo N. 2016. Data from: Parallel evolution of mound-building and grass-feeding in Australian nasute termites. GenBank Accession numbers KX011613-KX011851 and KX977324-KX977386. [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Sequence data have been uploaded to GenBank (accession numbers KX011613-KX011851 and KX977324-KX977386) [12].