Abstract
The intestine has long been studied as a model for adult stem cells due to the life-long self-renewal of the intestinal epithelium through the proliferation of the adult intestinal stem cells. Recent evidence suggests that the formation of adult intestinal stem cells in mammals takes place during the thyroid hormone-dependent neonatal period, also known as postembryonic development, which resembles intestinal remodeling during frog metamorphosis. Studies on the metamorphosis in Xenopus laevis have revealed that many members of the Sox family, a large family of DNA binding transcription factors, are upregulated in the intestinal epithelium during the formation and/or proliferation of the intestinal stem cells. Similarly, a number of Sox genes have been implicated in intestinal development and pathogenesis in mammals. Futures studies are needed to determine the expression and potential involvement of this important gene family in the development of the adult intestinal stem cells. These include the analyses of the expression and regulation of these and other Sox genes during postembryonic development in mammals as well as functional investigations in both mammals and amphibians by using the recently developed gene knockout technologies.
Keywords: Sox genes, thyroid hormone, metamorphosis, Xenopus, thyroid hormone receptor, stem cells, intestine, postembryonic development
Introduction
Like most many other tissues that are exposed to external environment, the vertebrate intestinal epithelium undergoes constant self-renewal throughout adult life. This is accomplished through the proliferation of the adult stem cells. In mammals, the stem cells are localized in the crypt of intestine while the differentiated cells, except the Paneth cells, are present along the villus of the crypt-villus axis [1–5]. As the stem cells proliferate in the crypt, their daughter cells migrate along the crypt-villus axis and gradually differentiate into different types of epithelial cells, leading to the replacement of the entire epithelium once every 1–6 days in mammals [1, 2, 5] [1, 2, 5]. Similar self-renewing structure is also present in other vertebrates, including anuran amphibians such as Xenopus laevis, where the epithelium is renewed once every 2 weeks [6]. Such an interesting self-renewing property has made intestine a valuable model system to study the property and functions of adult organ-specific stem cells. These studies have revealed many genes and signaling pathways important for intestinal development and cell renewal in the adult, among which include Wnt, Notch, and hedgehog pathways [4, 5, 7–9].
Increasing evidence suggest that members of the Sox gene family play critical roles in intestinal development and adult stem cell function. The Sox gene family consists of over two-dozen genes subdivided into 9 subgroups based on the presence of various structural domains [10]. They were first identified based on homology to the HMG box in mammalian testis determining factor SRY [11–13]. The Sox proteins are DNA-sequence specific transcription factors. A conserved HMG box is present in all Sox proteins and binds to specific DNA elements with a consensus of ATTGTT in the target genes [10–13]. Different other structural domains are present in different subgroups and enable Sox proteins to function as either transcriptional repressors or activators and interact with other proteins. Thus, not surprisingly, Sox genes are involved in diverse physiological and pathological processes. Here, we review some findings on the roles of Sox genes in the development of vertebrate intestine, with a particular focus on adult intestinal stem cells.
Vertebrate intestinal development and the formation of adult stem cells
In mammals, a functional intestine is formed during embryogenesis, which subsequently undergoes extensive maturation around birth to form the adult intestine [14–16]. During embryogenesis, the gut is developed from the definitive endoderm and the splanchnic lateral mesoderm, and subsequently differentiates into several digestive organs including stomach and intestine. In mouse, intestinal morphogenesis, in particular, the formation of the villus starts around embryonic day 14.5 (E14.5) [15, 16]. Subsequently, cell proliferation in the epithelium is gradually restricted to the intervillus region, where the invagination of the epithelium into the connective tissue gives rise to the formation of crypts after birth [15, 16]. (Fig. 1A). By about 3–4 weeks after birth, an adult intestine is formed with a villus-crypt axis, where the adult stem cells are localized in the crypts [14–16].
Fig. 1A.
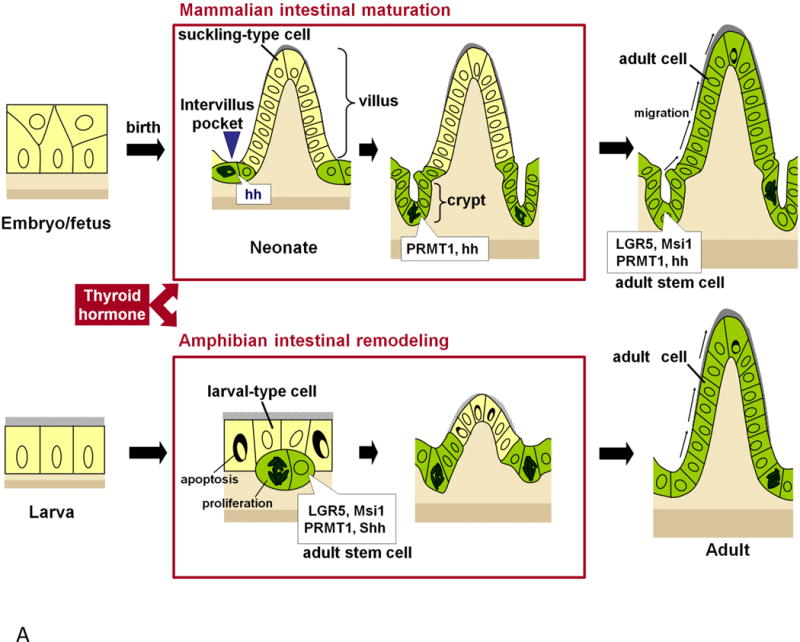
Mouse intestinal maturation (upper panel) resembles Xenopus metamorphic intestinal remodeling (lower panel). In both species, the adult stem cells are formed from the preexisting epithelial cells when the plasma thyroid hormone (T3) levels become high. After birth, cells in the intervillus region of the mouse intestine develop into adult stem cells expressing protein arginine methyltransferase 1 (PRMT1) and hedgehog (hh) (green cells with irregular-shaped dark nuclei) and invaginate into the underlying connective tissue to form the crypts. During Xenopus metamorphosis, some larval epithelial cells undergo dedifferentiation to become the adult stem cells that express high levels of PRMT1 and sonic hedgehog (Shh) (green cells with irregular-shaped dark nuclei). Subsequently, the descendants of these adult stem cells in both mouse and Xenopus replace the suckling-type or larval-type epithelial cells via active proliferation and differentiation to generate the adult epithelium possessing a self-renewal system (green cells). Modified after [14].
A similar developmental process takes place in the intestine of anuran amphibians. In Xenopus laevis and Xenopus tropicalis, perhaps the two best studied amphibian species, the intestine undergoes biphasic development, first forming a functional larval intestine by the end of embryogenesis, when a free-feeding tadpole is formed, and subsequent remodeling into the frog intestine during metamorphosis (Fig. 1B) [17, 18]. The tadpole or larval intestine has a simple tubular structure resembling the embryonic intestine in mammals. It is made of a single layer of larval epithelial cells surrounded by thin layers of connective tissue and muscles. There is a single epithelial fold in the tadpole intestine, the typhlosole, where connective tissue is abundant [17]. During metamorphosis, the larval epithelial cells undergo apoptosis and adult epithelial stem cells are formed de novo, and subsequently proliferate and differentiate to form the multiply folded adult epithelium with concurrent development of the muscles and connective tissue (Fig. 1B) [14, 17, 19–21]. While there is no further formation of the crypt-villus axis along the epithelial folds as in mammals, in the adult frog, the stem cells are localized at the bottom of the fold (the trough) while the differentiated epithelial cells at the tip of the fold (the crest) undergo apoptosis. Thus, the frog trough-crest axis resembles the crypt-villus axis in adult mammals.
Fig. 1B. Intestinal remodeling during Xenopus laevis metamorphosis.
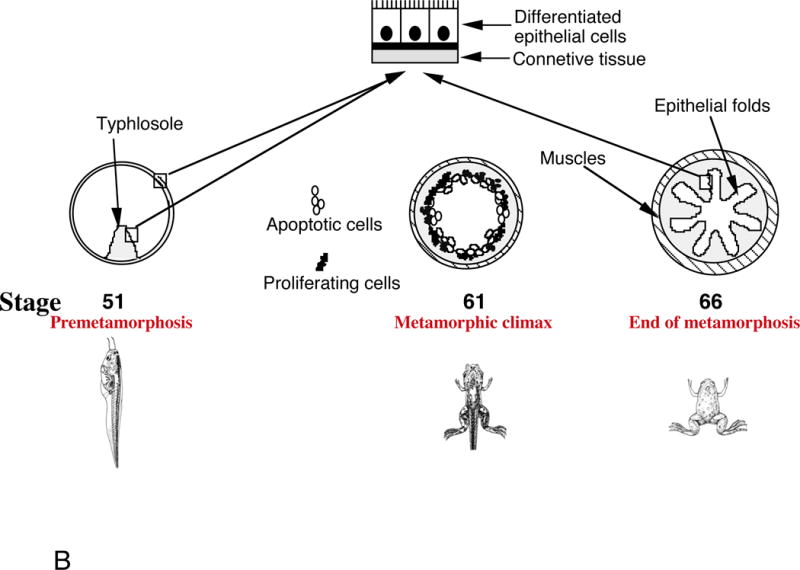
In premetamorphic tadpoles at stage 51, the intestine has a simple structure with only a single fold, the typhlosole. At the metamorphic climax around stage 61, the larval epithelial cells begin to undergo apoptosis, as indicated by the open circles. Concurrently, the proliferating adult stem cells are developed de novo from larval epithelial cells through dedifferentiation, as indicated by black dots. By the end of metamorphosis at stage 66, the newly differentiated adult epithelial cells form a multiply folded epithelium.
Regulation of intestinal development by thyroid hormone (T3)
Like many other organs, the intestine develops into the adult form, with well-established crypt-villus axis, during the postembryonic development, a period around birth in mammals [9, 14, 16, 21–23]. Interestingly, this period is also characterized by the presence of peak levels of T3 in the circulating plasma. In mouse, this corresponds to the first 3–4 weeks after birth with plasma T3 level peaking around 2 weeks after birth [24]. The intestine of newborn mice lacks crypts, and the crypts are formed as the T3 level rises in the plasma after birth [9, 14, 16, 23]. Importantly, T3 or T3 receptor (see below) deficiency leads to reduction in the number of epithelial cells along the crypt-villus axis and abnormal intestinal morphology [25–29], suggesting that T3 is important for the maturation of the mouse intestine.
Similarly, the remodeling of the larval intestine to the frog form takes place when plasma T3 level is high during amphibian metamorphosis [14, 17, 21]. In fact, many processes that occur during amphibian metamorphosis resemble those during mammalian postembryonic development [22, 30–32]. On the other hand, amphibian metamorphosis is absolutely dependent on T3 and takes place externally, independent of maternal influence. Thus, it can be easily manipulated in intact animals in vivo or even in organ or primary cell cultures by controlling the availability of T3 [22, 30, 33–35]. This has made amphibian metamorphosis an excellent model to study adult organ development in vertebrates.
Earlier studies have shown that T3-induces larval epithelial cells to undergo apoptosis and the formation of the adult intestinal stem cells [17, 21, 31, 36–38]. Importantly, there are no identifiable stem cells in the larval/tadpole epithelium that give rise to the adult epithelium. Instead, some larval epithelial cells, for yet unknown reason, undergo dedifferentiation induced by T3, and proliferate as clusters of cells or islets at the climax of natural metamorphosis or after prolonged T3 treatment (Fig. 2) [17, 39, 40]. These proliferating cell clusters express known molecular markers of mammalian adult intestinal stem cells, such as Lgr5 (Fig. 2) [40–42]. Thus, the formation of the adult intestine involves de novo development of adult stem cells, making intestinal metamorphosis a unique model to study how adult organ-specific stem cells are formed during vertebrate development.
Fig. 2. MGPY stains strongly the proliferating adult intestinal stem cells.
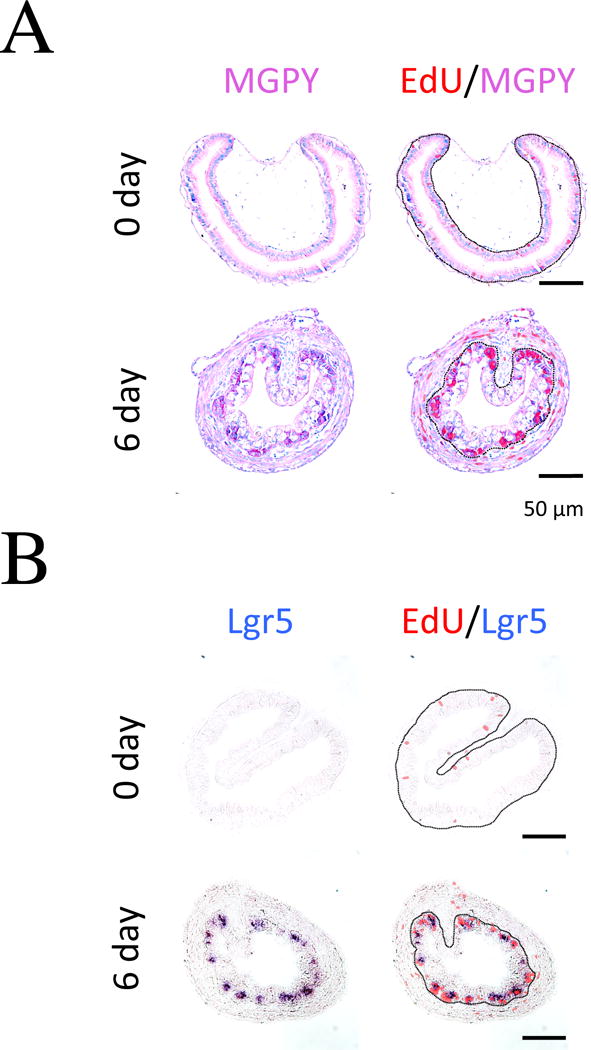
Premetamorphic stage 54 tadpoles treated with 10 nM T3 for 0, or 6 days were sacrificed one hour after injecting EdU. Cross-sections of the intestine from the resulting tadpoles were double-stained for EdU (5-ethynyl-2′-deoxyuridine) and with MGPY (methyl green pyronin Y, a mixture of methyl green, which stains DNA, and pyronin Y, which stains RNA [42, 145, 146]) (A) or for Edu and Lgr5 (in situ hybridization) (B). The approximate epithelium-mesenchyme boundary was drawn based on morphological differences between epithelial cells and mesenchyme cells in the pictures of the double-stained tissues (dotted lines). Note that the clusters (islets) of EdU labeled cells in the epithelium after 6 days of T3 treatment were strongly stained by MGPY and had high levels of Lgr5 mRNA, a well-established marker for adult intestinal stem cells in vertebrates. See [40] for more detail.
T3 is known to affect metamorphosis by regulating gene transcription through T3 receptors (TRs) [7, 43–50]. TRs are members of the nuclear hormone receptor superfamily and bind to specific DNA elements present in their target genes. In vitro and in vivo studies by using reporter genes have shown that for genes that are induced by T3, TRs mainly function as heterodimers with 9-cis retinoic acid receptors (RXRs), also members of the nuclear hormone receptor superfamily [51–54]. In the absence of T3, TR/RXR heterodimers repress target genes by recruiting corepressor complexes that contain histone deacetylases [47, 55–64]. When T3 is available, T3-bound TR/RXR recruits coactivator complexes containing various histone modification enzymes and chromatin remodeling proteins to alter local chromatin and activate transcription [46, 52, 55, 56, 58, 65–69]. Importantly, by using molecular and genetic approaches, we and others have shown that during Xenopus development, unliganded TR/RXR binds to target genes in premetamorphic tadpoles and recruits corepressor complexes, which are important to regulate the timing of metamorphosis [7, 46, 48–50, 70–72]. On the other hand, liganded TR is required to recruit coactivators to the endogenous target genes during metamorphosis to activate their expression and induce metamorphosis, including intestinal remodeling [43–46, 73–83].
Involvement of Sox genes during intestinal remodeling
Toward understanding how T3 regulates the intestinal stem cell development during metamorphosis, we have carried out microarray studies to identify genes that are regulated by T3 in the intestine [84–88]. Many genes that are regulated during intestinal remodeling were thus identified. Among them include genes of the Sox family (Table 1). Members of the Sox family are transcription factors that share a DNA binding domain made of a conserved HMG box [11–13]. They are classified into 9 groups. Members from different groups share little similarity except in the DNA-binding domain, while those within a group have similar domain organizations [10–13, 89]. Interestingly, among the genes regulated during intestinal metamorphosis include 11 Sox genes from 5 of the 9 groups (Table 1). Six of them are upregulated at stage 61, the climax of metamorphosis when adult stem cells are forming and proliferating [17, 40], implicating their potential involvement in these processes. These include the single regulated gene in the SoxB1 group (Sox3), all three of genes in the SoxC group (Sox 4, 11, 12), one each of the three regulated genes in SoxE or SoxF group (Sox9 or Sox17, respectively). In particular, Sox3, 4, and 12 are upregulated in the epithelium at the climax (stage 61), thus very likely directly involved in the developing adult stem cells, while it remains to be determined if the other three (Sox9, 11, 17) are upregulated in the epithelium.
Table 1.
T3-regulated Sox genes during intestinal metamorphosis in Xenopus laevis
| Gene | Subfamily | Regulation (relative to stage 54) | Intestinal tissues | References |
|---|---|---|---|---|
| Sox3 | SoxB1 | Up at stage 61 | Epithelium | [84, 85] |
| Sox4 | SoxC | Up at stage 61 | Both | [84, 85, 88] |
| Sox11 | SoxC | Up at stage 61 | Not known | [85, 88] |
| Sox12 | SoxC | Up at stage 61 | Both | [84] |
| Sox13 | SoxD | Up at stage 66 | Epithelium | [84, 88] |
| Sox8 | SoxE | Down at stage 61 | Non-epithelium | [84] |
| Sox9 | SoxE | Up at stage 61 | Not known | [85] |
| Sox10 | SoxE | Down at stage 61/66 | Epithelium | [84] |
| Up at stage 61/66 | Non-epithelium | |||
| Sox7 | SoxF | Down at stage 61/66 | Non-epithelium | [84, 85, 88] |
| Sox17 | SoxF | Up at stage 61 | Not known | [85, 88] |
| Sox18 | SoxF | Down at stage 61 | Non-epithelium | [84, 88] |
The single regulated gene in SoxD group, Sox13, is upregulated in the epithelium at stage 66, the end of metamorphosis, suggesting that it functions mainly in the frog intestinal epithelium. Three other genes, one in SoxE group (Sox8) and two in SoxF group (Sox7 and 18) were found to be down-regulated only in the non-epithelium (all intestinal tissues minus the epithelium) of the intestine at the climax of metamorphosis (stage 61) while the last gene, Sox10 in SoxE group, is downregulated in the epithelium but upregulated in the non-epithelium. Thus, these four genes are unlikely involved in stem cell formation and/or proliferation.
More detailed analyses have been carried out on the Sox3 gene. Sox3 belongs to the SoxB1 group that also include Sox1 and 2 [13]. All members of the SoxB1 group have been implicated in vertebrate neurogenesis during development [10, 13, 89–103]. Of particular interest, Sox2 is one of the four transcription factors that were initially found to be sufficient to reprogram mammalian fibroblasts into pluripotent stem cells [104]. In addition, in the central nervous system (CNS), all three SoxB1 genes appear to be required for stem-cell maintenance [10, 89, 91]. Thus, the upregulation of Sox3 during intestinal remodeling raises the possibility of its involvement in adult intestinal stem cell development and/or proliferation. As T3 is the causative agent of intestinal remodeling [8, 30, 32], T3 likely regulates the expression of Sox3. Indeed, T3 treatment of premetamorphic tadpoles leads to strong induction of Sox3 expression and this induction appears to be independent of new protein synthesis [105], suggesting that Sox3 is directly regulated by T3 at the transcription level via TRs. Consistently, several putative T3 response elements (TREs) are present upstream of the Sox3 transcription start site (Fig. 3A). In particular, one of them, TRE2 binds to TR/RXR heterodimers strongly in vitro and is required to mediate the activation of the Sox3 promoter by TR/RXR in the presence of T3 in reconstituted frog oocyte transcription system (Fig. 3B, C). Thus, TR/RXR likely binds to TRE2 in the tadpole intestine and activates Sox3 gene transcription during metamorphosis when T3 is present.
Fig. 3. A putative TRE in Xenopus Sox3 gene can mediate transcriptional activation by T3-bound TR/RXR in frog oocyte.
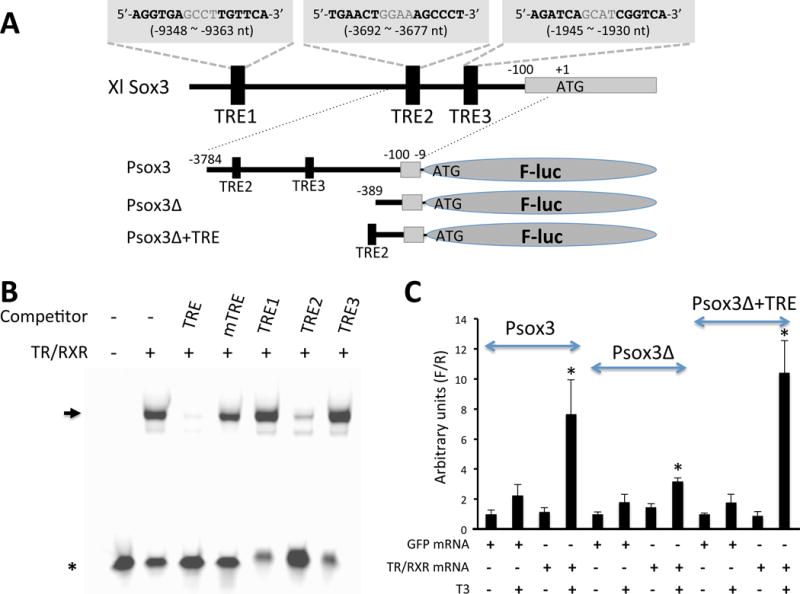
(A) Putative TREs and promoter constructs. Sox3 gene sequence was obtained from Xenopus laevis genome sequences at http://xenopus.lab.nig.ac.jp/blast.php and searched for TREs by using NHR Scan at http://nhrscan.genereg.net/cgi-bin/nhr_scan.cgi?rm=advanced. Three putative TREs were found and are listed above the schematic diagram of the Sox3 gene with their positions relative to the first nucleotide of the start codon (designated as “+1”). Three promoter constructs, the full length Sox3 promoter including the putative TRE2 and TRE3 (Psox3), a truncated version of Sox3 promoter (Psox3Δ), and the truncated version of Sox3 promoter with the TRE2 (Psox3Δ+TRE) inserted immediately upstream of it, were generated to drive the firefly luciferase expression in pGL4 vector (Promega). TRE: thyroid hormone response element; F-luc: firefly luciferase gene.
(B) TRE2 binds to TR/RXR in vitro. Double strand DNA oligos containing the putative TREs shown in (A) were used in competitive electrophoretic mobility shift assay (EMSA) against the infrared (IR) dye IR700 (LI-COR, Lincoln, NE)-labeled, well-characterized TRE of Xenopus laevis TRβ gene in the presence of in vitro translated TR/RXR proteins. Unlabeled TRE of the Xenopus laevis TRβ gene (TRE) and a mutant version of TRE of Xenopus laevis TRβ gene (mTRE) known to lack binding to TR/RXR were used as the positive and negative control, respectively [147, 148]. All unlabeled oligos were used in 100 times excess over the labeled IR700-TRE. Note that only TRE and TRE2 competed effectively, suggesting that TRE2, but not TRE1 or TRE3, binds to TR/RXR specifically.
(C) Sox3 promoter can be activated by liganded TR/RXR in frog oocyte. Transcription assay was done in Xenopus laevis oocytes where the cytoplasm of stage VI oocytes were injected with 460 pg per oocyte of TR and RXR mRNA mixture or GFP mRNA. 2 hours later, the firefly luciferase reporter constructs shown in (A) (34.5 pg per oocyte) and the phRG-TK (Promega) expressing Renilla luciferase as an internal control (34.5 pg per oocyte) were coinjected into the nuclei of the oocytes. After incubation at 18°C overnight in the presence or absence of 100 nM T3, 5 oocytes were collected per sample and lysed in 75 μl of 1×Passive Lysis Buffer (Promega) for dual luciferase assay by following the manufacturer’s protocol. The relative expression of firefly luciferase to Renilla luciferase (F/R) was determined with the F/R value for oocytes injected with GFP mRNA instead of TR/RXR in the absence of T3 set as 1. Note that the full-length promoter was activated by TR/RXR in the presence of T3. This activation was drastically reduced when the TRE sequences were deleted and was restored when TRE2 was inserted into the truncated promoter, suggesting that TRE2 is capable of mediating T3 induction. Each data point shows the average of 5 samples with the standard error. Statistical analysis was done through ANOVA with Tukey’s Multiple Component Test. *: p<0.05. (L. Fu and Y.-B. Shi, unpublished observations).
Developmentally, Sox3 gene has little expression in the intestine before or after metamorphosis but highly upregulated at the climax of metamorphosis (stage 58–64, Fig. 4A). In particular, its mRNA level peaks at stage 62, when most of the cells in the epithelium are proliferating adult stem cells [17, 39, 40]. Furthermore, Sox3 is exclusively expressed in the intestinal epithelium but not in the rest of the intestinal tissues (non-epithelium, Fig. 4B). Finally, in situ hybridization analysis indicates that Sox3 is expressed in the proliferating adult epithelial stem cells at the climax metamorphosis [105]. These findings strongly support a role of Sox3 in either the formation and/or proliferation of adult intestinal stem cells during metamorphosis.
Fig. 4. Tissue-specific developmental regulation of Sox3 in the intestine during Xenopus laevis metamorphosis.
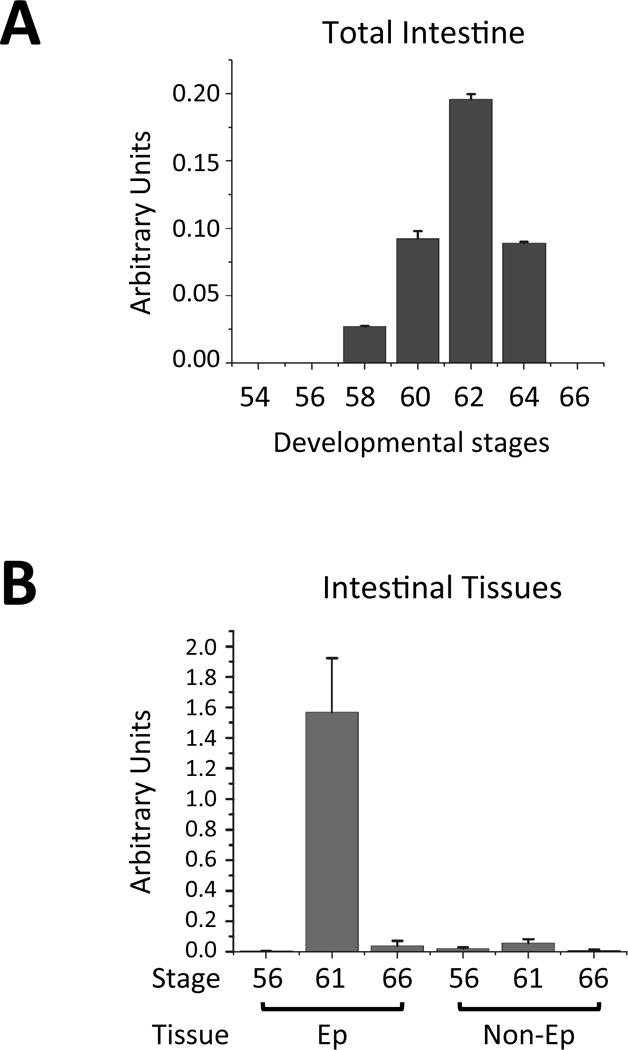
(A) Sox3 is highly expressed only during metamorphosis. Total intestinal RNA at different stages was analyzed by qRT-PCR. Note that little Sox3 expression was found either before (stage 54–56) or at the end of metamorphosis (stage 66).
(B) Sox3 is expressed only in the intestinal epithelium. Total RNA was isolated from intestinal epithelium (Ep) and the rest of the intestine (non-Ep) at different stages of development, 56 (premetamorphosis), 61 (climax), and 66 (end of metamorphosis), and analyzed by qRT-PCR. Note that Sox3 was highly expressed only in the Ep at the climax of metamorphosis when stem cells were forming or proliferating. See [105] for details.
While similar analyses have not been done on other Sox genes regulated during intestinal metamorphosis, the tissue- and developmental stage-dependent regulation of different Sox genes during intestinal metamorphosis (Table 1) argues for different roles of different Sox genes in the intestine. It is very likely that the other five Sox genes (Sox4, 9, 11, 12, 17) that are upregulated at the climax of metamorphosis (stage 61) (Table 1) are involved in adult stem cell development, while the rest (Sox7, Sox8, Sox10, Sox13, and Sox18) likely function in the larval and/or adult intestine but not in the stem cell development during metamorphosis as inferred from their spatiotemporal regulation patterns.
Sox genes during mammalian intestinal development and pathogenesis
Increasing evidence points to the involvement of Sox genes in mammalian intestinal development and a few excellent reviews have been published on this subject [106–108]. One of the most studied in the gastrointestinal tract is Sox9, a member of the SoxE subfamily. Sox9 was first described as the gene whose haploinsufficiency leads to Campomelic Dysplasia with symptoms including XY sex reversal, skeletal defects and pancreatic endocrine defects [108–113]. It has subsequently been shown to be important for the development of many organs and tissues, including the intestine [108]. During mouse development, Sox9 is expressed throughout duodenal epithelium as early as embryonic day 13.5 (E13.5) and subsequently restricted to the proliferating epithelial cells, the Paneth and enteroendocrine cells around E18.5, and finally only in the crypt of the adult intestine [114]. Sox9 is regulated by Wnt pathway, which is known to be important for intestinal stem cells, and in turn represses Cdx2 and Muc2, two genes involved in epithelial differentiation [114], implicating a role in adult intestinal stem cell function. On the other hand, conditional knockout of the Sox9 in the intestinal epithelium led to increased proliferation [115], suggesting possible compensation by related genes. Consistent with the expression of Sox9 in the Paneth cells, the conditional knockout resulted in the loss of Paneth cells in the crypts and altered intestinal morphology [115, 116].
Sox9 appears to function in a dose-dependent manner, as first revealed by the causative role of Sox9 haploinsufficiency in Campomelic Dysplasia [109, 111, 112]. GFP reporter labeling studies indicate that Sox9 is expressed mostly in two types of cells in the crypt, with high and low levels of Sox9 expression, respectively [117, 118]. The cells with high levels of Sox9 expression are differentiated enteroendocrine cells [117]. The crypt cells with low levels of Sox9 expression also express well-known adult intestinal stem cell markers such as Lgr5 and Musashi [117]. Thus, Sox9 function in the intestine is dose-dependent.
Another Sox gene, Sox17, has been shown to be expressed in the intestine in both human and mouse [119–121]. Importantly, Sox17 knockout in mouse results in the loss of definitive gut endoderm and no homozygous Sox17 knockout embryos were found after E10.5 or at birth [122]. Furthermore, in chimeras, Sox17-null ES cells can contribute to ectodermal and mesodermal tissues, few of them colonize the foregut and hindgut endoderm [122]. In addition, studies by using an in vitro differentiation system of embryonic stem cells have shown that Sox17 is important for late-stage differentiation of the extraembryonic endoderm [123]. Thus, Sox17 plays a critical role in early intestinal development.
Sox2, a member of the SoxB1 subfamily [13] and perhaps the best-known Sox gene due to it being one of the four transcription factors that were initially found to be sufficient to reprogram fibroblasts into pluripotent stem cells is Sox2 [104], has also been implicated in intestinal development [124]. Earlier studies have shown that like other members of the SoxB1 subfamily, Sox2 plays roles in inducing neural progenitor cell formation during embryonic development in vertebrates and are required for stem-cell maintenance in the central nervous system (CNS) [13, 90–93, 95, 97, 99, 103]. More recently, Kuzmichev et al. used an inducible system to express Sox2 in Lgr5+ intestinal stem cells in mice and observed that Sox2 expression increased stem cells and repressed the expression of Cdx2, a master regulator of endodermal identity [124]. Mechanistically, they showed that Sox2 affects intestinal stem cell identity by regulating the expression of Sox21, another member of the SoxB gene family, in cell-autonomous manner in intestinal stem cells and that Sox21 in turn repress the expression of Cdx2 [124].
Additionally, Sox4 [121], Sox7 [125], Sox10 [126], and Sox18 [127] are known to be expressed in mouse and/or human intestine. But their spatiotemporal expression profiles are not known and no functional studies have been carried out.
Given their involvement in intestinal development and/or function, it is not surprising that increasing studies have revealed the participation of Sox genes in intestinal diseases [106, 107]. Sox9 appears to have a tumor suppressive function. Sox9 mRNA and protein are strongly expressed in a number of colon cancer cell lines compared to a non-intestinal epithelial cell line [114]. On the other hand, overexpressing Sox9 increases apoptosis in colon cancer cell lines in vitro by inhibiting the expression of the carcinoembryonic antigen, which has anti-apoptotic effect in colon cancers [128, 129]. Similarly, Sox9-deficiency in mice and colorectal carcinoma leads to increased expression of claudin-7, which in turn promotes a loss of tumor cell polarization and contributes to tumorigenesis [130]. Finally, Sox9 knockout in mice resulted in not only the loss of Paneth cells in the crypt but also general hyperplasia and local crypt dysplasia, accompanied by increased expression of Wnt pathway target genes in the intestine [116].
Similarly, Sox7 is frequently down-regulated in human colorectal cancer cell lines and in primary colorectal tumor tissues [131]. Restoration of Sox7 expression induces colorectal cancer cell apoptosis and inhibits cell proliferation and colony formation [131]. Sox7 may function as a tumor suppressor in part by inhibiting Wnt signaling pathway as it can significantly reduce the Wnt/β-catenin stimulated gene expression [125, 131]. Finally, Sox4 [132, 133] and Sox17 [134, 135] also have altered expression in intestinal tumors, although their roles are not clear at the present time.
Conclusion
The constant self-renewal of the intestinal epithelium throughout adult life through stem cells residing in intestinal crypts and increasing incidence of intestinal diseases, especially colon cancer have made intestine an important and well studied model for understanding organogenesis and stem cell function. Given the importance of adult organ-specific stem cells in organ homeostasis, understanding the formation of such stem cells is critical for the potential use of stem cells in tissue-repair and regeneration. Increasing evidence suggest that the formation of the adult intestinal stem cells in mammals occurs during postembryonic development when T3 levels peaks in vertebrates [9, 14, 16, 21–23, 25–29, 31, 136–138]. However, little is known about if and how T3 affects the formation of such stem cells during postembryonic development in mammals, in part due to the difficulty to study this process.
Frog metamorphosis mimics postembryonic development in mammals, including the formation of the adult intestine, which resembles the maturation of the mouse intestine around birth [14, 16, 21–23, 31, 137, 138]. As indicated above, both intestinal metamorphosis and postembryonic intestinal maturation in mammals are dependent on T3. Importantly, studies on the transcriptional repressor Blimp-1 (B-lymphocyte induced maturation protein 1) [14, 16, 23, 139] and histone methyltransferase PRMT1 (protein arginine methyltransferase 1) [140] suggest that adult intestinal stem cells are formed after birth in mouse during the postembryonic period when T3 levels are high, resembling the formation of adult intestinal stem cells during Xenopus metamorphosis. Thus, adult intestinal stem cells are likely formed in both mammals and frogs via conserved molecular mechanisms during this T3-dependent developmental period [14–16]. The ability to manipulate Xenopus intestinal metamorphosis coupled with advances in global gene expression analyses has allowed the discovery of many genes regulated during the development of the adult intestinal stem cells, including many members of the Sox gene family.
The Sox genes are particularly attractive as candidate regulators of adult stem cell development in vertebrates. Many Sox genes have been implicated to play critical roles in stem cells during embryogenesis and in adult organs [10, 89]. They encode DNA-binding transcription factors. This makes it easy to identify their direct downstream targets through global analyses such as RNA-seq and ChIP (chromatin immunoprecipitation assay)-seq, which would facilitate the determination of the molecular mechanisms underlying their roles in stem cells. Furthermore, many of the Sox genes are capable of interacting with the Wnt signaling pathway, which has long been shown to be important for adult intestinal stem cells [4, 5, 106].
Studies in the Xenopus model system reveal that Sox3 is a direct target of T3, the causative agent of adult intestinal stem cell formation during Xenopus metamorphosis [30] and that Sox3 is highly upregulated exclusively in the adult stem cells during intestinal metamorphosis [105]. These suggest that Sox3 play an important role during the early stages of intestinal metamorphosis, likely the formation and/proliferation of the adult stem cells. Such a role is consistent with earlier findings that all three member of the SoxB1 family, Sox1, Sox2, and Sox3, are highly conserved in vertebrates and important for inducing neural progenitor cell formation during early embryonic development in vertebrates and for stem-cell maintenance in the central nervous system (CNS) [10, 89, 91]. Additionally, Sox2 is one of the four transcription factors what were first shown to be required and sufficient for reprogramming mammalian fibroblasts into pluripotent stem cells is Sox2 [104, 141]. Clearly, functional studies by using approaches such as gain-of-function via transgenesis [142] or loss-of-function via knockdown/knockout [143, 144] should help to determine the exact roles of Sox3 and the other Sox genes regulated by T3 (Table 1) in adult stem cells development during Xenopus metamorphosis.
The involvement of Sox genes in the development of adult intestinal stem cells in mammals remains to be investigated. It would be interesting to determine if Sox genes, especially those implicated in intestinal development in amphibians (table 1) and mammals, such as Sox9 and Sox17, are expressed and/or regulated by T3 during the postembryonic period (around birth) in mammals when adult intestinal stem cells are formed. More importantly, it would be critical to investigate if these Sox genes play any roles in the development of the adult stem cells during this period and whether their functions are conserved in vertebrates.
Acknowledgments
Grant Support: This work was supported by the intramural Research Program of NICHD, NIH
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURE STATEMENT: The authors have nothing to disclose
References
- 1.MacDonald WC, Trier JS, Everett NB. Cell proliferation and migration in the stomach, duodenum, and rectum of man: Radioautographic studies. Gastroenterology. 1964;46:405–417. [PubMed] [Google Scholar]
- 2.Toner PG, Carr KE, Wyburn GM. The Digestive System: An Ultrastructural Atlas and Review. London: Butterworth; 1971. [Google Scholar]
- 3.Cheng H, Leblond CP. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. III. Entero-endocrine cells. Am J Anat. 1974;141(4):503–519. doi: 10.1002/aja.1001410405. [DOI] [PubMed] [Google Scholar]
- 4.Sancho E, Eduard Batlle E, Clevers H. Signaling pathways in intestinal development and cancer. Annu Rev Cell DevBiol. 2004;20:695–723. doi: 10.1146/annurev.cellbio.20.010403.092805. [DOI] [PubMed] [Google Scholar]
- 5.van der Flier LG, Clevers H. Stem Cells, Self-Renewal, and Differentiation in the Intestinal Epithelium. Annu Rev Physiol. 2009;71:241–260. doi: 10.1146/annurev.physiol.010908.163145. [DOI] [PubMed] [Google Scholar]
- 6.McAvoy JW, Dixon KE. Cell proliferation and renewal in the small intestinal epithelium of metamorphosing and adult Xenopus laevis. J Exp Zool. 1977;202:129–138. [Google Scholar]
- 7.Wen L, Hasebe T, Miller TC, Ishizuya-Oka A, Shi YB. A requirement for hedgehog signaling in thyroid hormone-induced postembryonic intestinal remodeling. Cell Biosci. 2015;5:13. doi: 10.1186/s13578-015-0004-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun G, Fu L, Shi Y-B. Epigenetic regulation of thyroid hormone-induced adult intestinal stem cell development during anuran metamorphosis. Cell Biosci. 2014;4:73. doi: 10.1186/2045-3701-4-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun G, Shi Y-B. Thyroid hormone regulation of adult intestinal stem cell development: Mechanisms and evolutionary conservations. Int J Biol Sci. 2012;8:1217–1224. doi: 10.7150/ijbs.5109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kamachi Y, Kondoh H. Sox proteins: regulators of cell fate specification and differentiation. Development. 2013;140(20):4129–4144. doi: 10.1242/dev.091793. [DOI] [PubMed] [Google Scholar]
- 11.Lovell-Badge R. The early history of the Sox genes. Int J Biochem Cell Biol. 2010;42(3):378–380. doi: 10.1016/j.biocel.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 12.Sekido R, Lovell-Badge R. Sex determination and SRY: down to a wink and a nudge? Trends Genet. 2009;25(1):19–29. doi: 10.1016/j.tig.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 13.Bowles J, Schepers G, Koopman P. Phylogeny of the SOX family of developmental transcription factors based on sequence and structural indicators. Dev Biol. 2000;227(2):239–255. doi: 10.1006/dbio.2000.9883. [DOI] [PubMed] [Google Scholar]
- 14.Ishizuya-Oka A, Shi YB. Evolutionary insights into postembryonic development of adult intestinal stem cells. Cell Biosci. 2011;1(1):37. doi: 10.1186/2045-3701-1-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crosnier C, Stamataki D, Lewis J. Organizing cell renewal in the intestine: stem cells, signals and combinatorial control. NATURE REVIEWS | GENETICS. 2006;7:349–359. doi: 10.1038/nrg1840. [DOI] [PubMed] [Google Scholar]
- 16.Harper J, Mould A, Andrews RM, Bikoff EK, Robertson EJ. The transcriptional repressor Blimp1/Prdm1 regulates postnatal reprogramming of intestinal enterocytes. Proc Natl Acad Sci U S A. 2011;108(26):10585–10590. doi: 10.1073/pnas.1105852108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi Y-B, Ishizuya-Oka A. Biphasic intestinal development in amphibians: Embryogensis and remodeling during metamorphosis. Current Topics in Develop Biol. 1996;32:205–235. doi: 10.1016/s0070-2153(08)60429-9. [DOI] [PubMed] [Google Scholar]
- 18.Sterling J, Fu L, Matsuura K, Shi Y-B. Cytological and morphological analyses reveal distinct features of intestinal development during Xenopus tropicalis metamorphosis. PLoS One. 2012;7:e47407, 47401–47410. doi: 10.1371/journal.pone.0047407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ishizuya-Oka A, Hasebe T, Buchholz DR, Kajita M, Fu L, Shi YB. Origin of the adult intestinal stem cells induced by thyroid hormone in Xenopus laevis. Faseb J. 2009;23:2568–2575. doi: 10.1096/fj.08-128124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schreiber AM, Cai L, Brown DD. Remodeling of the intestine during metamorphosis of Xenopus laevis. Proc Natl Acad Sci U S A. 2005;102(10):3720–3725. doi: 10.1073/pnas.0409868102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shi YB, Hasebe T, Fu L, Fujimoto K, Ishizuya-Oka A. The development of the adult intestinal stem cells: Insights from studies on thyroid hormone-dependent amphibian metamorphosis. Cell Biosci. 2011;1(1):30. doi: 10.1186/2045-3701-1-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tata JR. Gene expression during metamorphosis: an ideal model for post-embryonic development. Bioessays. 1993;15(4):239–248. doi: 10.1002/bies.950150404. [DOI] [PubMed] [Google Scholar]
- 23.Muncan V, Heijmans J, Krasinski SD, Buller NV, Wildenberg ME, Meisner S, Radonjic M, Stapleton KA, Lamers WH, Biemond I, van den Bergh Weerman MA, O’Carroll D, Hardwick JC, Hommes DW, van den Brink GR. Blimp1 regulates the transition of neonatal to adult intestinal epithelium. Nat Commun. 2011;2:452. doi: 10.1038/ncomms1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Friedrichsen S, Christ S, Heuer H, Schäfer MKH, Mansouri A, Bauer K, Visser TJ. Regulation of iodothyronine deiodinases in the Pax8−/− mouse model of congenital hypothyroidism. Endocrinology. 2003;144:777–784. doi: 10.1210/en.2002-220715. [DOI] [PubMed] [Google Scholar]
- 25.Plateroti M, Gauthier K, Domon-Dell C, Freund JN, Samarut J, Chassande O. Functional interference between thyroid hormone receptor alpha (TRalpha) and natural truncated TRDeltaalpha isoforms in the control of intestine development. Mol Cell Biol. 2001;21(14):4761–4772. doi: 10.1128/MCB.21.14.4761-4772.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Flamant F, Poguet AL, Plateroti M, Chassande O, Gauthier K, Streichenberger N, Mansouri A, Samarut J. Congenital hypothyroid Pax8(−/−) mutant mice can be rescued by inactivating the TRalpha gene. Mol Endocrinol. 2002;16(1):24–32. doi: 10.1210/mend.16.1.0766. [DOI] [PubMed] [Google Scholar]
- 27.Kress E, Rezza A, Nadjar J, Samarut J, Plateroti M. The frizzled-related sFRP2 gene is a target of thyroid hormone receptor alpha1 and activates beta-catenin signaling in mouse intestine. J Biol Chem. 2009;284(2):1234–1241. doi: 10.1074/jbc.M806548200. [DOI] [PubMed] [Google Scholar]
- 28.Plateroti M, Chassande O, Fraichard A, Gauthier K, Freund JN, Samarut J, Kedinger M. Involvement of T3Ralpha- and beta-receptor subtypes in mediation of T3 functions during postnatal murine intestinal development. Gastroenterology. 1999;116(6):1367–1378. doi: 10.1016/s0016-5085(99)70501-9. [DOI] [PubMed] [Google Scholar]
- 29.Plateroti M, Kress E, Mori JI, Samarut J. Thyroid hormone receptor alpha1 directly controls transcription of the beta-catenin gene in intestinal epithelial cells. Mol Cell Biol. 2006;26(8):3204–3214. doi: 10.1128/MCB.26.8.3204-3214.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shi Y-B. Amphibian Metamorphosis: From morphology to molecular biology. New York: John Wiley & Sons, Inc; 1999. [Google Scholar]
- 31.Hasebe T, Fu L, Miller TC, Zhang Y, Shi YB, Ishizuya-Oka A. Thyroid hormone-induced cell-cell interactions are required for the development of adult intestinal stem cells. Cell Biosci. 2013;3(1):18. doi: 10.1186/2045-3701-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shi Y-B, Ishizuya-Oka A. Thyroid hormone regulation of apoptotic tissue remodeling: Implications from molecular analysis of amphibian metamorphosis. Progress in Nucleic Acid Research and Molecular Biology. 2001;65:53–100. doi: 10.1016/s0079-6603(00)65002-x. [DOI] [PubMed] [Google Scholar]
- 33.Su Y, Shi Y, Shi Y-B. Cyclosporin A But not FK506 Inhibits Thyroid Hormone-Induced Apoptosis in Xenopus Tadpole Intestinal Epithelium. FASEB J. 1997;11:559–565. doi: 10.1096/fasebj.11.7.9212079. [DOI] [PubMed] [Google Scholar]
- 34.Su Y, Shi Y, Stolow M, Shi Y-B. Thyroid hormone induces apoptosis in primary cell cultures of tadpole intestine: cell type specificity and effects of extracellular matrix. J Cell Biol. 1997;139:1533–1543. doi: 10.1083/jcb.139.6.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ishizuya-Oka A, Shimozawa A. Induction of metamorphosis by thyroid hormone in anuran small intestine cultured organotypically in vitro. In Vitro Cell Dev Biol. 1991;27A(11):853–857. doi: 10.1007/BF02630987. [DOI] [PubMed] [Google Scholar]
- 36.Ishizuya-Oka A, Shimozawa A. Connective tissue is involved in adult epithelial development of the small intestine during anuran metamorphosis in vitro. Roux’s Arch Dev Biol. 1992;201:322–329. doi: 10.1007/BF00592113. [DOI] [PubMed] [Google Scholar]
- 37.Ishizuya-Oka A, Shimozawa A. Inductive action of epithelium on differentiation of intestinal connective tissue of Xenopus laevis tadpoles during metamorphosis in vitro. Cell Tissue Res. 1994;277(3):427–436. doi: 10.1007/BF00300215. [DOI] [PubMed] [Google Scholar]
- 38.Hasebe T, Buchholz DR, Shi YB, Ishizuya-Oka A. Epithelial-connective tissue interactions induced by thyroid hormone receptor are essential for adult stem cell development in the Xenopus laevis intestine. Stem Cells. 2011;29(1):154–161. doi: 10.1002/stem.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ishizuya-Oka A, Shi YB. Thyroid hormone regulation of stem cell development during intestinal remodeling. Mol Cell Endocrinol. 2008;288(1–2):71–78. doi: 10.1016/j.mce.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 40.Okada M, Wen L, Miller TC, Su D, Shi YB. Molecular and cytological analyses reveal distinct transformations of intestinal epithelial cells during Xenopus metamorphosis. Cell Biosci. 2015;5:74. doi: 10.1186/s13578-015-0065-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun G, Hasebe T, Fujimoto K, Lu R, Fu L, Matsuda H, Kajita M, Ishizuya-Oka A, Shi YB. Spatio-temporal expression profile of stem cell-associated gene LGR5 in the intestine during thyroid hormone-dependent metamorphosis in Xenopus laevis. PLoS One. 2010;5(10):e13605. doi: 10.1371/journal.pone.0013605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ishizuya-Oka A, Shimizu K, Sakakibara S, Okano H, Ueda S. Thyroid hormone-upregulated expression of Musashi-1 is specific for progenitor cells of the adult epithelium during amphibian gastrointestinal remodeling. J Cell Sci. 2003;116(Pt 15):3157–3164. doi: 10.1242/jcs.00616. [DOI] [PubMed] [Google Scholar]
- 43.Buchholz DR, Tomita A, Fu L, Paul BD, Shi Y-B. Transgenic analysis reveals that thyroid hormone receptor is sufficient to mediate the thyroid hormone signal in frog metamorphosis. Mol Cell Biol. 2004;24:9026–9037. doi: 10.1128/MCB.24.20.9026-9037.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Buchholz DR, Hsia VS-C, Fu L, Shi Y-B. A dominant negative thyroid hormone receptor blocks amphibian metamorphosis by retaining corepressors at target genes. Mol Cell Biol. 2003;23:6750–6758. doi: 10.1128/MCB.23.19.6750-6758.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schreiber AM, Das B, Huang H, Marsh-Armstrong N, Brown DD. Diverse developmental programs of Xenopus laevis metamorphosis are inhibited by a dominant negative thyroid hormone receptor. PNAS. 2001;98:10739–10744. doi: 10.1073/pnas.191361698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shi Y-B. Dual functions of thyroid hormone receptors in vertebrate development: the roles of histone-modifying cofactor complexes. Thyroid. 2009;19:987–999. doi: 10.1089/thy.2009.0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shi YB, Matsuura K, Fujimoto K, Wen L, Fu L. Thyroid hormone receptor actions on transcription in amphibia: The roles of histone modification and chromatin disruption. Cell Biosci. 2012;2(1):42. doi: 10.1186/2045-3701-2-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yen PM. Unliganded TRs regulate growth and developmental timing during early embryogenesis: evidence for a dual function mechanism of TR action. Cell Biosci. 2015;5:8. doi: 10.1186/2045-3701-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Choi J, Suzuki KI, Sakuma T, Shewade L, Yamamoto T, Buchholz DR. Unliganded thyroid hormone receptor alpha regulates developmental timing via gene repression as revealed by gene disruption in Xenopus tropicalis. Endocrinology. 2015;156:735–744. doi: 10.1210/en.2014-1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sachs LM. Unliganded thyroid hormone receptor function: amphibian metamorphosis got TALENs. Endocrinology. 2015;156(2):409–410. doi: 10.1210/en.2014-2016. [DOI] [PubMed] [Google Scholar]
- 51.Lazar MA. Thyroid hormone receptors: multiple forms, multiple possibilities. Endocr Rev. 1993;14(2):184–193. doi: 10.1210/edrv-14-2-184. [DOI] [PubMed] [Google Scholar]
- 52.Yen PM. Physiological and molecular basis of thyroid hormone action. Physiol Rev. 2001;81(3):1097–1142. doi: 10.1152/physrev.2001.81.3.1097. [DOI] [PubMed] [Google Scholar]
- 53.Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P. The nuclear receptor superfamily: the second decade. Cell. 1995;83(6):835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tsai MJ, O’Malley BW. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Ann Rev Biochem. 1994;63:451–486. doi: 10.1146/annurev.bi.63.070194.002315. [DOI] [PubMed] [Google Scholar]
- 55.Burke LJ, Baniahmad A. Co-repressors 2000. FASEB J. 2000;14(13):1876–1888. doi: 10.1096/fj.99-0943rev. [DOI] [PubMed] [Google Scholar]
- 56.Jones PL, Shi Y-B. N-CoR-HDAC corepressor complexes: Roles in transcriptional regulation by nuclear hormone receptors. In: Workman JL, editor. Current Topics in Microbiology and Immunology: Protein Complexes that Modify Chromatin. Vol. 274. Berlin: Springer-Verlag; 2003. pp. 237–268. [DOI] [PubMed] [Google Scholar]
- 57.Glass CK, Rosenfeld MG. The coregulator exchange in transcriptional functions of nuclear receptors. Genes Dev. 2000;14(2):121–141. [PubMed] [Google Scholar]
- 58.Zhang J, Lazar MA. The mechanism of action of thyroid hormones. Annu Rev Physiol. 2000;62:439–466. doi: 10.1146/annurev.physiol.62.1.439. [DOI] [PubMed] [Google Scholar]
- 59.Yoon H-G, Chan DW, Huang ZQ, Li J, Fondell JD, Qin J, Wong J. Purification and functional characterization of the human N-CoR complex: the roles of HDAC3, TBL1 and TBLR1. Embo J. 2003;22:1336–1346. doi: 10.1093/emboj/cdg120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang J, Kalkum M, Chait BT, Roeder RG. The N-CoR-HDAC3 nuclear receptor corepressor complex inhibits the JNK pathway through the integral subunit GPS2. Mol Cell. 2002;9:611–623. doi: 10.1016/s1097-2765(02)00468-9. [DOI] [PubMed] [Google Scholar]
- 61.Ishizuka T, Lazar MA. The N-CoR/histone deacetylase 3 complex is required for repression by thyroid hormone receptor. Mol Cell Biol. 2003;23:5122–5131. doi: 10.1128/MCB.23.15.5122-5131.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guenther MG, Lane WS, Fischle W, Verdin E, Lazar MA, Shiekhattar R. A core SMRT corepressor complex containing HDAC3 and TBL1, a WD40-repeat protein linked to deafness. Genes & Devel. 2000;14:1048–1057. [PMC free article] [PubMed] [Google Scholar]
- 63.Li J, Wang J, Wang J, Nawaz Z, Liu JM, Qin J, Wong J. Both corepressor proteins SMRT and N-CoR exist in large protein complexes containing HDAC3. Embo J. 2000;19:4342–4350. doi: 10.1093/emboj/19.16.4342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jones PL, Sachs LM, Rouse N, Wade PA, Shi YB. Multiple N-CoR complexes contain distinct histone deacetylases. J Biol Chem. 2001;276(12):8807–8811. doi: 10.1074/jbc.C000879200. [DOI] [PubMed] [Google Scholar]
- 65.Ito M, Roeder RG. The TRAP/SMCC/Mediator complex and thyroid hormone receptor function. Trends Endocrinol Metab. 2001;12(3):127–134. doi: 10.1016/s1043-2760(00)00355-6. [DOI] [PubMed] [Google Scholar]
- 66.Rachez C, Freedman LP. Mechanisms of gene regulation by vitamin D(3) receptor: a network of coactivator interactions. Gene. 2000;246(1–2):9–21. doi: 10.1016/s0378-1119(00)00052-4. [DOI] [PubMed] [Google Scholar]
- 67.Jepsen K, Rosenfeld MG. Biological roles and mechanistic actions of co-repressor complexes. J Cell Sci. 2002;115(Pt 4):689–698. doi: 10.1242/jcs.115.4.689. [DOI] [PubMed] [Google Scholar]
- 68.Rachez C, Freedman LP. Mediator complexes and transcription. Curr Opin Cell Biol. 2001;13(3):274–280. doi: 10.1016/s0955-0674(00)00209-x. [DOI] [PubMed] [Google Scholar]
- 69.Hu X, Lazar MA. Transcriptional Repression by Nuclear Hormone Receptors. TEM. 2000;11(1):6–10. doi: 10.1016/s1043-2760(99)00215-5. [DOI] [PubMed] [Google Scholar]
- 70.Tomita A, Buchholz DR, Shi Y-B. Recruitment of N-CoR/SMRT-TBLR1 corepressor complex by unliganded thyroid hormone receptor for gene repression during frog development. Mol Cell Biol. 2004;24:3337–3346. doi: 10.1128/MCB.24.8.3337-3346.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sachs LM, Jones PL, Havis E, Rouse N, Demeneix BA, Shi Y-B. N-CoR recruitment by unliganded thyroid hormone receptor in gene repression during Xenopus laevis development. Mol Cell Biol. 2002;22:8527–8538. doi: 10.1128/MCB.22.24.8527-8538.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sato Y, Buchholz DR, Paul BD, Shi Y-B. A role of unliganded thyroid hormone receptor in postembryonic development in Xenopus laevis. Mechanisms of Development. 2007;124:476–488. doi: 10.1016/j.mod.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Brown DD, Cai L. Amphibian metamorphosis. Dev Biol. 2007;306(1):20–33. doi: 10.1016/j.ydbio.2007.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Buchholz DR, Paul BD, Fu L, Shi YB. Molecular and developmental analyses of thyroid hormone receptor function in Xenopus laevis, the African clawed frog. Gen Comp Endocrinol. 2006;145(1):1–19. doi: 10.1016/j.ygcen.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 75.Nakajima K, Yaoita Y. Dual mechanisms governing muscle cell death in tadpole tail during amphibian metamorphosis. Dev Dyn. 2003;227:246–255. doi: 10.1002/dvdy.10300. [DOI] [PubMed] [Google Scholar]
- 76.Denver RJ, Hu F, Scanlan TS, Furlow JD. Thyroid hormone receptor subtype specificity for hormone-dependent neurogenesis in Xenopus laevis. Dev Biol. 2009;326(1):155–168. doi: 10.1016/j.ydbio.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 77.Bagamasbad P, Howdeshell KL, Sachs LM, Demeneix BA, Denver RJ. A role for basic transcription element-binding protein 1 (BTEB1) in the autoinduction of thyroid hormone receptor beta. J Biol Chem. 2008;283:2275–2285. doi: 10.1074/jbc.M709306200. [DOI] [PubMed] [Google Scholar]
- 78.Schreiber AM, Mukhi S, Brown DD. Cell-cell interactions during remodeling of the intestine at metamorphosis in Xenopus laevis. Dev Biol. 2009;331(1):89–98. doi: 10.1016/j.ydbio.2009.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Matsuda H, Paul BD, Choi CY, Hasebe T, Shi Y-B. Novel functions of protein arginine methyltransferase 1 in thyroid hormone receptor-mediated transcription and in the regulation of metamorphic rate in Xenopus laevis. Mol Cell Biol. 2009;29:745–757. doi: 10.1128/MCB.00827-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Paul BD, Buchholz DR, Fu L, Shi Y-B. Tissue- and gene-specific recruitment of steroid receptor coactivator-3 by thyroid hormone receptor during development. J Biol Chem. 2005;280:27165–27172. doi: 10.1074/jbc.M503999200. [DOI] [PubMed] [Google Scholar]
- 81.Paul BD, Fu L, Buchholz DR, Shi Y-B. Coactivator recruitment is essential for liganded thyroid hormone receptor to initiate amphibian metamorphosis. Mol Cell Biol. 2005;25:5712–5724. doi: 10.1128/MCB.25.13.5712-5724.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Paul BD, Buchholz DR, Fu L, Shi Y-B. SRC-p300 coactivator complex is required for thyroid hormone induced amphibian metamorphosis. J Biol Chem. 2007;282:7472–7481. doi: 10.1074/jbc.M607589200. [DOI] [PubMed] [Google Scholar]
- 83.Havis E, Sachs LM, Demeneix BA. Metamorphic T3-response genes have specific co-regulator requirements. EMBO Reports. 2003;4:883–888. doi: 10.1038/sj.embor.embor908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sun G, Heimeier RA, Fu L, Hasebe T, Das B, Ishizuya-Oka A, Shi Y-B. Expression Profiling of Intestinal Tissues Implicates Tissue-Specific Genes and Pathways Essential for Thyroid Hormone-Induced Adult Stem Cell Development. Endocrinology. 2013;154(11):4396–4407. doi: 10.1210/en.2013-1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Heimeier RA, Das B, Buchholz DR, Fiorentino M, Shi YB. Studies on Xenopus laevis intestine reveal biological pathways underlying vertebrate gut adaptation from embryo to adult. Genome Biol. 2010;11(5):R55. doi: 10.1186/gb-2010-11-5-r55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Heimeier RA, Das B, Buchholz DR, Shi YB. The xenoestrogen bisphenol A inhibits postembryonic vertebrate development by antagonizing gene regulation by thyroid hormone. Endocrinology. 2009;150(6):2964–2973. doi: 10.1210/en.2008-1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Das B, Heimeier RA, Buchholz DR, Shi YB. Identification of direct thyroid hormone response genes reveals the earliest gene regulation programs during frog metamorphosis. J Biol Chem. 2009;284:34167–34178. doi: 10.1074/jbc.M109.066084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Buchholz DR, Heimeier RA, Das B, Washington T, Shi Y-B. Pairing morphology with gene expression in thyroid hormone-induced intestinal remodeling and identification of a core set of TH-induced genes across tadpole tissues. Dev Biol. 2007;303:576–590. doi: 10.1016/j.ydbio.2006.11.037. [DOI] [PubMed] [Google Scholar]
- 89.Sarkar A, Hochedlinger K. The sox family of transcription factors: versatile regulators of stem and progenitor cell fate. Cell Stem Cell. 2013;12(1):15–30. doi: 10.1016/j.stem.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Archer TC, Jin J, Casey ES. Interaction of Sox1, Sox2, Sox3 and Oct4 during primary neurogenesis. Dev Biol. 2011;350(2):429–440. doi: 10.1016/j.ydbio.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wegner M, Stolt CC. From stem cells to neurons and glia: a Soxist’s view of neural development. Trends Neurosci. 2005;28(11):583–588. doi: 10.1016/j.tins.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 92.Kelberman D, de Castro SC, Huang S, Crolla JA, Palmer R, Gregory JW, Taylor D, Cavallo L, Faienza MF, Fischetto R, Achermann JC, Martinez-Barbera JP, Rizzoti K, Lovell-Badge R, Robinson IC, Gerrelli D, Dattani MT. SOX2 plays a critical role in the pituitary, forebrain, and eye during human embryonic development. J Clin Endocrinol Metab. 2008;93(5):1865–1873. doi: 10.1210/jc.2007-2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fauquier T, Rizzoti K, Dattani M, Lovell-Badge R, Robinson IC. SOX2-expressing progenitor cells generate all of the major cell types in the adult mouse pituitary gland. Proc Natl Acad Sci U S A. 2008;105(8):2907–2912. doi: 10.1073/pnas.0707886105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rizzoti K, Lovell-Badge R. SOX3 activity during pharyngeal segmentation is required for craniofacial morphogenesis. Development. 2007;134(19):3437–3448. doi: 10.1242/dev.007906. [DOI] [PubMed] [Google Scholar]
- 95.Kelberman D, Rizzoti K, Avilion A, Bitner-Glindzicz M, Cianfarani S, Collins J, Chong WK, Kirk JM, Achermann JC, Ross R, Carmignac D, Lovell-Badge R, Robinson IC, Dattani MT. Mutations within Sox2/SOX2 are associated with abnormalities in the hypothalamo-pituitary-gonadal axis in mice and humans. J Clin Invest. 2006;116(9):2442–2455. doi: 10.1172/JCI28658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Woods KS, Cundall M, Turton J, Rizotti K, Mehta A, Palmer R, Wong J, Chong WK, Al-Zyoud M, El-Ali M, Otonkoski T, Martinez-Barbera JP, Thomas PQ, Robinson IC, Lovell-Badge R, Woodward KJ, Dattani MT. Over- and underdosage of SOX3 is associated with infundibular hypoplasia and hypopituitarism. Am J Hum Genet. 2005;76(5):833–849. doi: 10.1086/430134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kiernan AE, Pelling AL, Leung KK, Tang AS, Bell DM, Tease C, Lovell-Badge R, Steel KP, Cheah KS. Sox2 is required for sensory organ development in the mammalian inner ear. Nature. 2005;434(7036):1031–1035. doi: 10.1038/nature03487. [DOI] [PubMed] [Google Scholar]
- 98.Rizzoti K, Brunelli S, Carmignac D, Thomas PQ, Robinson IC, Lovell-Badge R. SOX3 is required during the formation of the hypothalamo-pituitary axis. Nat Genet. 2004;36(3):247–255. doi: 10.1038/ng1309. [DOI] [PubMed] [Google Scholar]
- 99.Hoffmann SA, Hos D, Kuspert M, Lang RA, Lovell-Badge R, Wegner M, Reiprich S. Stem cell factor Sox2 and its close relative Sox3 have differentiation functions in oligodendrocytes. Development. 2014;141(1):39–50. doi: 10.1242/dev.098418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sutton E, Hughes J, White S, Sekido R, Tan J, Arboleda V, Rogers N, Knower K, Rowley L, Eyre H, Rizzoti K, McAninch D, Goncalves J, Slee J, Turbitt E, Bruno D, Bengtsson H, Harley V, Vilain E, Sinclair A, Lovell-Badge R, Thomas P. Identification of SOX3 as an XX male sex reversal gene in mice and humans. J Clin Invest. 2011;121(1):328–341. doi: 10.1172/JCI42580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bylund M, Andersson E, Novitch BG, Muhr J. Vertebrate neurogenesis is counteracted by Sox1–3 activity. Nat Neurosci. 2003;6(11):1162–1168. doi: 10.1038/nn1131. [DOI] [PubMed] [Google Scholar]
- 102.Rogers CD, Harafuji N, Archer T, Cunningham DD, Casey ES. Xenopus Sox3 activates sox2 and geminin and indirectly represses Xvent2 expression to induce neural progenitor formation at the expense of non-neural ectodermal derivatives. Mech Dev. 2009;126(1–2):42–55. doi: 10.1016/j.mod.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pevny L, Placzek M. SOX genes and neural progenitor identity. Curr Opin Neurobiol. 2005;15(1):7–13. doi: 10.1016/j.conb.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 104.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 105.Sun G, Fu L, Wen L, Shi YB. Activation of sox3 gene by thyroid hormone in the developing adult intestinal stem cell during Xenopus metamorphosis. Endocrinology. 2014;155(12):5024–5032. doi: 10.1210/en.2014-1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kormish JD, Sinner D, Zorn AM. Interactions between SOX factors and Wnt/beta-catenin signaling in development and disease. Dev Dyn. 2010;239(1):56–68. doi: 10.1002/dvdy.22046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gracz AD, Magness ST. Sry-box (Sox) transcription factors in gastrointestinal physiology and disease. Am J Physiol Gastrointest Liver Physiol. 2011;300(4):G503–515. doi: 10.1152/ajpgi.00489.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Belo J, Krishnamurthy M, Oakie A, Wang R. The role of SOX9 transcription factor in pancreatic and duodenal development. Stem Cells Dev. 2013;22(22):2935–2943. doi: 10.1089/scd.2013.0106. [DOI] [PubMed] [Google Scholar]
- 109.Wagner T, Wirth J, Meyer J, Zabel B, Held M, Zimmer J, Pasantes J, Bricarelli FD, Keutel J, Hustert E, Wolf U, Tommerup N, Schempp W, Scherer G. Autosomal sex reversal and campomelic dysplasia are caused by mutations in and around the SRY-related gene SOX9. Cell. 1994;79(6):1111–1120. doi: 10.1016/0092-8674(94)90041-8. [DOI] [PubMed] [Google Scholar]
- 110.Piper K, Ball SG, Keeling JW, Mansoor S, Wilson DI, Hanley NA. Novel SOX9 expression during human pancreas development correlates to abnormalities in Campomelic dysplasia. Mech Dev. 2002;116(1–2):223–226. doi: 10.1016/s0925-4773(02)00145-4. [DOI] [PubMed] [Google Scholar]
- 111.Foster JW, Dominguez-Steglich MA, Guioli S, Kwok C, Weller PA, Stevanovic M, Weissenbach J, Mansour S, Young ID, Goodfellow PN, et al. Campomelic dysplasia and autosomal sex reversal caused by mutations in an SRY-related gene. Nature. 1994;372(6506):525–530. doi: 10.1038/372525a0. [DOI] [PubMed] [Google Scholar]
- 112.Bi W, Huang W, Whitworth DJ, Deng JM, Zhang Z, Behringer RR, de Crombrugghe B. Haploinsufficiency of Sox9 results in defective cartilage primordia and premature skeletal mineralization. Proc Natl Acad Sci U S A. 2001;98(12):6698–6703. doi: 10.1073/pnas.111092198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gordon CT, Tan TY, Benko S, Fitzpatrick D, Lyonnet S, Farlie PG. Long-range regulation at the SOX9 locus in development and disease. J Med Genet. 2009;46(10):649–656. doi: 10.1136/jmg.2009.068361. [DOI] [PubMed] [Google Scholar]
- 114.Blache P, van de Wetering M, Duluc I, Domon C, Berta P, Freund JN, Clevers H, Jay P. SOX9 is an intestine crypt transcription factor, is regulated by the Wnt pathway, and represses the CDX2 and MUC2 genes. J Cell Biol. 2004;166(1):37–47. doi: 10.1083/jcb.200311021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mori-Akiyama Y, van den Born M, van Es JH, Hamilton SR, Adams HP, Zhang J, Clevers H, de Crombrugghe B. SOX9 is required for the differentiation of paneth cells in the intestinal epithelium. Gastroenterology. 2007;133(2):539–546. doi: 10.1053/j.gastro.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 116.Bastide P, Darido C, Pannequin J, Kist R, Robine S, Marty-Double C, Bibeau F, Scherer G, Joubert D, Hollande F, Blache P, Jay P. Sox9 regulates cell proliferation and is required for Paneth cell differentiation in the intestinal epithelium. J Cell Biol. 2007;178(4):635–648. doi: 10.1083/jcb.200704152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Formeister EJ, Sionas AL, Lorance DK, Barkley CL, Lee GH, Magness ST. Distinct SOX9 levels differentially mark stem/progenitor populations and enteroendocrine cells of the small intestine epithelium. Am J Physiol Gastrointest Liver Physiol. 2009;296(5):G1108–1118. doi: 10.1152/ajpgi.00004.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gracz AD, Ramalingam S, Magness ST. Sox9 expression marks a subset of CD24-expressing small intestine epithelial stem cells that form organoids in vitro. Am J Physiol Gastrointest Liver Physiol. 2010;298(5):G590–600. doi: 10.1152/ajpgi.00470.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Katoh M. Molecular cloning and characterization of human SOX17. Int J Mol Med. 2002;9(2):153–157. [PubMed] [Google Scholar]
- 120.Sinner D, Rankin S, Lee M, Zorn AM. Sox17 and beta-catenin cooperate to regulate the transcription of endodermal genes. Development. 2004;131(13):3069–3080. doi: 10.1242/dev.01176. [DOI] [PubMed] [Google Scholar]
- 121.Sinner D, Kordich JJ, Spence JR, Opoka R, Rankin S, Lin SC, Jonatan D, Zorn AM, Wells JM. Sox17 and Sox4 differentially regulate beta-catenin/T-cell factor activity and proliferation of colon carcinoma cells. Mol Cell Biol. 2007;27(22):7802–7815. doi: 10.1128/MCB.02179-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kanai-Azuma M, Kanai Y, Gad JM, Tajima Y, Taya C, Kurohmaru M, Sanai Y, Yonekawa H, Yazaki K, Tam PP, Hayashi Y. Depletion of definitive gut endoderm in Sox17-null mutant mice. Development. 2002;129(10):2367–2379. doi: 10.1242/dev.129.10.2367. [DOI] [PubMed] [Google Scholar]
- 123.Shimoda M, Kanai-Azuma M, Hara K, Miyazaki S, Kanai Y, Monden M, Miyazaki J. Sox17 plays a substantial role in late-stage differentiation of the extraembryonic endoderm in vitro. J Cell Sci. 2007;120(Pt 21):3859–3869. doi: 10.1242/jcs.007856. [DOI] [PubMed] [Google Scholar]
- 124.Kuzmichev AN, Kim SK, D’Alessio AC, Chenoweth JG, Wittko IM, Campanati L, McKay RD. Sox2 acts through Sox21 to regulate transcription in pluripotent and differentiated cells. Curr Biol. 2012;22(18):1705–1710. doi: 10.1016/j.cub.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 125.Takash W, Canizares J, Bonneaud N, Poulat F, Mattei MG, Jay P, Berta P. SOX7 transcription factor: sequence, chromosomal localisation, expression, transactivation and interference with Wnt signalling. Nucleic Acids Res. 2001;29(21):4274–4283. doi: 10.1093/nar/29.21.4274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pusch C, Hustert E, Pfeifer D, Sudbeck P, Kist R, Roe B, Wang Z, Balling R, Blin N, Scherer G. The SOX10/Sox10 gene from human and mouse: sequence, expression, and transactivation by the encoded HMG domain transcription factor. Hum Genet. 1998;103(2):115–123. doi: 10.1007/s004390050793. [DOI] [PubMed] [Google Scholar]
- 127.Saitoh T, Katoh M. Expression of human SOX18 in normal tissues and tumors. Int J Mol Med. 2002;10(3):339–344. [PubMed] [Google Scholar]
- 128.Jay P, Berta P, Blache P. Expression of the carcinoembryonic antigen gene is inhibited by SOX9 in human colon carcinoma cells. Cancer Res. 2005;65(6):2193–2198. doi: 10.1158/0008-5472.CAN-04-1484. [DOI] [PubMed] [Google Scholar]
- 129.Wirth T, Soeth E, Czubayko F, Juhl H. Inhibition of endogenous carcinoembryonic antigen (CEA) increases the apoptotic rate of colon cancer cells and inhibits metastatic tumor growth. Clin Exp Metastasis. 2002;19(2):155–160. doi: 10.1023/a:1014566127493. [DOI] [PubMed] [Google Scholar]
- 130.Darido C, Buchert M, Pannequin J, Bastide P, Zalzali H, Mantamadiotis T, Bourgaux JF, Garambois V, Jay P, Blache P, Joubert D, Hollande F. Defective claudin-7 regulation by Tcf-4 and Sox-9 disrupts the polarity and increases the tumorigenicity of colorectal cancer cells. Cancer Res. 2008;68(11):4258–4268. doi: 10.1158/0008-5472.CAN-07-5805. [DOI] [PubMed] [Google Scholar]
- 131.Zhang Y, Huang S, Dong W, Li L, Feng Y, Pan L, Han Z, Wang X, Ren G, Su D, Huang B, Lu J. SOX7, down-regulated in colorectal cancer, induces apoptosis and inhibits proliferation of colorectal cancer cells. Cancer Lett. 2009;277(1):29–37. doi: 10.1016/j.canlet.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 132.Andersen CL, Christensen LL, Thorsen K, Schepeler T, Sorensen FB, Verspaget HW, Simon R, Kruhoffer M, Aaltonen LA, Laurberg S, Orntoft TF. Dysregulation of the transcription factors SOX4, CBFB and SMARCC1 correlates with outcome of colorectal cancer. Br J Cancer. 2009;100(3):511–523. doi: 10.1038/sj.bjc.6604884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Reichling T, Goss KH, Carson DJ, Holdcraft RW, Ley-Ebert C, Witte D, Aronow BJ, Groden J. Transcriptional profiles of intestinal tumors in Apc(Min) mice are unique from those of embryonic intestine and identify novel gene targets dysregulated in human colorectal tumors. Cancer Res. 2005;65(1):166–176. [PubMed] [Google Scholar]
- 134.Zhang W, Glockner SC, Guo M, Machida EO, Wang DH, Easwaran H, Van Neste L, Herman JG, Schuebel KE, Watkins DN, Ahuja N, Baylin SB. Epigenetic inactivation of the canonical Wnt antagonist SRY-box containing gene 17 in colorectal cancer. Cancer Res. 2008;68(8):2764–2772. doi: 10.1158/0008-5472.CAN-07-6349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Paoni NF, Feldman MW, Gutierrez LS, Ploplis VA, Castellino FJ. Transcriptional profiling of the transition from normal intestinal epithelia to adenomas and carcinomas in the APCMin/+ mouse. Physiol Genomics. 2003;15(3):228–235. doi: 10.1152/physiolgenomics.00078.2003. [DOI] [PubMed] [Google Scholar]
- 136.Lopez-Juarez A, Remaud S, Hassani Z, Jolivet P, Pierre Simons J, Sontag T, Yoshikawa K, Price J, Morvan-Dubois G, Demeneix BA. Thyroid hormone signaling acts as a neurogenic switch by repressing Sox2 in the adult neural stem cell niche. Cell Stem Cell. 2012;10(5):531–543. doi: 10.1016/j.stem.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 137.Kress E, Samarut J, Plateroti M. Thyroid hormones and the control of cell proliferation or cell differentiation: paradox or duality? Mol Cell Endocrinol. 2009;313(1–2):36–49. doi: 10.1016/j.mce.2009.08.028. [DOI] [PubMed] [Google Scholar]
- 138.Sirakov M, Plateroti M. The thyroid hormones and their nuclear receptors in the gut: from developmental biology to cancer. Biochim Biophys Acta. 2011;1812(8):938–946. doi: 10.1016/j.bbadis.2010.12.020. [DOI] [PubMed] [Google Scholar]
- 139.Mould AW, Morgan MA, Nelson AC, Bikoff EK, Robertson EJ. Blimp1/Prdm1 Functions in Opposition to Irf1 to Maintain Neonatal Tolerance during Postnatal Intestinal Maturation. PLoS Genet. 2015;11(7):e1005375. doi: 10.1371/journal.pgen.1005375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Matsuda H, Shi YB. An essential and evolutionarily conserved role of protein arginine methyltransferase 1 for adult intestinal stem cells during postembryonic development. Stem Cells. 2010;28(11):2073–2083. doi: 10.1002/stem.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Pei D. Regulation of pluripotency and reprogramming by transcription factors. J Biol Chem. 2009;284(6):3365–3369. doi: 10.1074/jbc.R800063200. [DOI] [PubMed] [Google Scholar]
- 142.Fu L, Buchholz D, Shi YB. Novel double promoter approach for identification of transgenic animals: A tool for in vivo analysis of gene function and development of gene-based therapies. Mol Reprod Dev. 2002;62(4):470–476. doi: 10.1002/mrd.10137. [DOI] [PubMed] [Google Scholar]
- 143.Lei Y, Guo X, Deng Y, Chen Y, Zhao H. Generation of gene disruptions by transcription activator-like effector nucleases (TALENs) in Xenopus tropicalis embryos. Cell Biosci. 2013;3(1):21. doi: 10.1186/2045-3701-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wang F, Shi Z, Cui Y, Guo X, Shi YB, Chen Y. Targeted gene disruption in Xenopus laevis using CRISPR/Cas9. Cell Biosci. 2015;5:15. doi: 10.1186/s13578-015-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ishizuya-Oka A, Ueda S, Damjanovski S, Li Q, Liang VC, Shi Y-B. Anteroposterior gradient of epithelial transformation during amphibian intestinal remodeling: immunohistochemical detection of intestinal fatty acid-binding protein. Dev Biol. 1997;192(1):149–161. doi: 10.1006/dbio.1997.8749. [DOI] [PubMed] [Google Scholar]
- 146.Ishizuya-Oka A, Ueda S. Apoptosis and cell proliferation in the Xenopus small intestine during metamorphosis. Cell Tissue Res. 1996;286(3):467–476. doi: 10.1007/s004410050716. [DOI] [PubMed] [Google Scholar]
- 147.Ranjan M, Wong J, Shi YB. Transcriptional repression of Xenopus TR beta gene is mediated by a thyroid hormone response element located near the start site. J Biol Chem. 1994;269(40):24699–24705. [PubMed] [Google Scholar]
- 148.Wong J, Shi Y-B. Coordinated regulation of and transcriptional activation by Xenopus thyroid hormone and retinoid X receptors. J Biol Chem. 1995;270:18479–18483. doi: 10.1074/jbc.270.31.18479. [DOI] [PubMed] [Google Scholar]


