Abstract
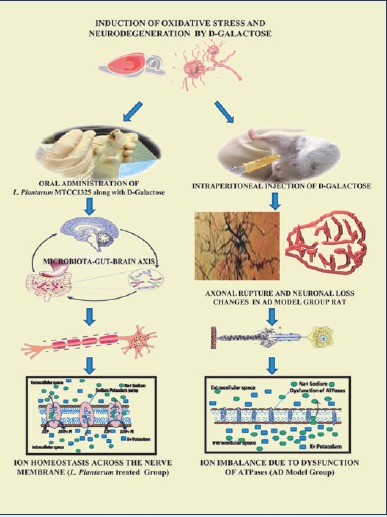
Introduction: Alzheimer’s disease (AD) is a neurodegenerative disorder, clinically characterized by memory dysfunction and progressive loss of cognition. No curative therapeutic or drug is available for the complete cure of this disease. The present study was aimed to evaluate the efficacy of Lactobacillus plantarum MTCC1325 in ATPases activity in the selected brain regions of rats induced with Alzheimer’s.
Methods: For the study, 48 healthy Wistar rats were divided into four groups: group I as control group, group II as AD model (AD induced by intraperitoneal injection of D-Galactose, 120 mg/kg body weight for 6 weeks), group III as normal control rats which were orally administered only with L. plantarum MTCC1325 for 60 days, and group IV where the AD-induced rats simultaneously received oral treatment of L. plantarum MTCC1325 (10ml/kg body weight, 12×108 CFU/mL) for 60 days. The well known membrane bound transport enzymes including Na+, K+-ATPases, Ca2+-ATPases, and Mg2+-ATPases were assayed in the selected brain regions of hippocampus and cerebral cortex in all four groups of rats at selected time intervals.
Results: Chronic injection of D-Galactose caused lipid peroxidation, oxidative stress, and mitochondrial dysfunction leading to the damage of neurons in the brain, finally bringing a significant decrease (-20%) in the brain total membrane bound ATPases over the controls. Contrary to this, treatment of AD-induced rats with L. plantarum MTCC1325 reverted all the constituents of ATPase enzymes to near normal levels within 30 days.
Conclusion: Lactobacillus plantarum MTCC1325 exerted a beneficial action on the entire ATPases system in AD-induced rat brain by delaying neurodegeneration.
Keywords: Alzheimer’s disease, ATPases, D-Galactose, Lactobacillus plantarum
Introduction
Alzheimer’s disease (AD) is the most prevalent neurodegenerative disorder in the aged people across the globe. Worldwide, nearly 44 million people have Alzheimer’s related dementia and it is estimated that more than 4 million and 5.3 million individuals have Alzheimer’s in India and the United States, respectively (http://www.alz.org/in/dementia-alzheimers-en.asp; http://www.alz.org/alzheimers_disease_trajectory.asp). It is projected that more than 13.5 million individuals will have AD by the year 2050.1 AD is clinically characterized by the brain pathological hallmarks such as neurofabrillary tangles, dystrophic neuritis, and β amyloid plaques derived from the amyloid precursor proteins.2 Neurotoxicity of amyloid plaques increases oxidative stress and leads to the generation of reactive oxygen species involved in mitochondrial dysfunction and damage of neuronal membranes, lipids, proteins, and nucleic acids.3 Prolonged supplementation of D-Galactose induces oxidative stress, mitochondrial dysfunction, and intracellular damage of neurons, accelerating ageing, and influencing age-related cognitive decline in experimental animals.4
Na+, K+-ATPase is a membrane-bound transport enzyme, which regulates the ionic concentration gradient across the cell membranes by permitting the entry of two K+ ions into the cells and exit of three Na+ ions out of the cells. Further, it maintains the membrane potential, cell volume, trans membrane flux of Ca2+, and excitatory neurotransmitters.5 Recent research findings demonstrated that frontal cortex and cerebellum regions of AD patient’s brain showed increased levels of Na+and K+ion concentrations and Amyloid beta 25-35 peptide in primary astrocytes which eventually led to the reduced levels of membrane transporter Na+, K+-ATPases.6 This observation gives us a clue that elevation of ATPases-associated energy metabolism might help in controlling the AD progression to some extent.
Lactic acid bacteria (LAB), considered as GRAS (generally recognized as safe),7 are the best studied beneficial microorganisms for the prevention of diarrhea, lactose intolerance, treating ulcer, infectious diseases prevention, restoring the composition of gut microbiome and introducing beneficial functions to host through immune, neuro modulation.8,9 They also possess a wide variety of activities like anticancer, antioxidant, antidiabetic, antiobesity, and antihyperlipidemic effects.10 Recent studies have found that the L. plantarum versus pentosus showed protective effect against memory deficit in AD-induced mice by D-Galactose and scopolamine.11,12 Further, another study reported that L. Plantarum NDC75017 improves the learning and memory ability in aging rats.13 Previous reports proved that strong antioxidants increases the Na+, K+-ATPases activities by decreasing AChE (Acetyl cholinesterase) levels and improves the cognition by enhancing cholinergic transmission.14
All the above cited research findings prompted us to undertake the present work to investigate the potential therapeutic qualities of L. plantarum MTCC1325 on the functional activities of membrane-bound ATPases in the brain tissue of D-Galactose-induced AD model rats.
Materials and methods
Chemicals
D-Galactose, TCA (trichloroacetic acid), ATP (adenosine triphosphate) and MRS (de Man, Rogosa and Sharpe) broth were procured from Hi-Media Laboratories (Mumbai, India). Nacl (sodium chloride), Mgcl2 (magnesium chloride), Kcl (pottasium chloride), and H2SO4 (sulphuric acid) were obtained from Qualigen Chemicals (Mumbai, India). ANSA (1-amino 2-naphthol 4-sulphuric acid), Tris buffer, ammonium molybdate, and Sodium bisulphate were purchased from CDH chemicals (New Delhi, India).
Lactic Acid Bacteria: The pure culture of L. plantarum MTCC1325 was obtained from the IMTech Culture Collection Center, Chandigarh, India.
Experimental design
A total of 48 healthy adult male Wistar rats (3 months old, weighing 180 ± 20 g) were procured from Sri Venkateswara Enterprisers, Bangalore and maintained in the laboratory in the controlled conditions of 28 ± 2°C, 12 h light/12 h dark. The rats were fed with standard pellet diet and water ad libitum throughout the experiment period.
The rats were divided into 4 groups, each consisting of six animals.
Group I (control group): Rats were subcutaneously injected with saline (1 mL/kg body weight).
Group-II (AD model group): Rats received D-galactose (120 mg/kg body weight) through intraperitoneal injection.
Group III (LP: Lactobacillus Plantarum group): Rats were injected with saline for first six weeks and with L .plantarum from seventh week onwards (10 mL/kg body weight of rat; 12×108 CFU/mL) for 60 days.
Group IV (AD+LP [protective group]): Rats received D-Galactose (120 mg/kg body weight) for six weeks then treated with L. plantarum (10 mL/kg body weight of rat; 12×108 CFU/mL) from seventh week onwards for another 60 days.
Preparation of Lactobacillus plantarum culture for treatment
Lactobacillus plantarum MTCC1325 was provisionally isolated and identified from the saukraut fermented food product by Stephenson and Rowatt in 1947 and it was found that this strain had the ability to produce Acetylcholine Neurotransmitter.15 A loop full of pure culture of L. Plantarum was inoculated in MRS broth and incubated at 30°C in orbital shaker until to get log phase. One millilitre of this active log phase culture was serially diluted up to 10-9 dilution in drinking water. The Dilution 10-8containing 12×108 CFU/mL of L. plantarum MTCC1325 was selected for oral administration. Each group of rats were orally administered with L. plantarum MTCC1325 (10 mL/kg body weight) through stainless steel gastric gavage.13
Isolation of tissues
After 6 weeks of AD induction, the animals were treated with L. plantarum for a period of 60 days. The animals were kept fasting for 12 h before sacrificing and on the selected days of experimentation (30th and 60th days), they were decapitated and the brain regions of hippocampus (HP) and cerebral cortex (CC) from each animal were rapidly isolated, thoroughly washed with ice cold saline and then stored in -80°C for biochemical analysis.
Biochemical analysis
HP and CC regions of brain tissue samples isolated from all the groups of experimental rats were used for the estimation of membrane bound transport ATPases using following methods. The total ATPases and Mg2+-ATPases were estimated by the method of Tirri et al.16
Total ATPases
One percent homogenates of different regions of rat brain were prepared in 0.25 M ice cold sucrose solution. The reaction mixture in a final volume of 2.6 mL contained 0.5 mL of tris buffer (0.13 M; pH 7.4), 0.4 mL of substrate ATP (4.0 μ moles), 0.5 mL of MgCl2 (0.05 M), 0.5 mL of NaCl (0.05 M),0.5 mL of KCl (0.05 M), and 0.2 mL of homogenate. The contents were incubated at 37°C for 15 min and the reaction was terminated by adding 1.5 mL of 10% TCA. The contents were centrifuged and the released inorganic phosphate was estimated in the supernatant fraction. Sodium-potassium ATPases can be obtained by subtracting the Mg2+-ATPases from the total ATPases.
Mg2+ -ATPases
One percent homogenates of different regions of rat brain were prepared in 0.25 M ice-cold sucrose solution. The reaction mixture in a final volume of 1.6 mL contained 0.5 mL of tris buffer (0.13 M; pH 7.4), 0.4 mL of substrate ATP (4.0 μ moles), 0.5 mL of MgCl2 (0.05 M), 10 μ moles of ouabain, and 0.2 mL of homogenate. The contents were incubated at 37°C for 15 min and the reaction was terminated by adding 1.5 mL of 10% TCA. Zero time controls were maintained by adding TCA prior to the addition of homogenate. The contents were centrifuged at 1000 X g for 15 min and the inorganic phosphate was estimated in the supernatant fraction.
Ca2+-ATPases
Ca2+-ATPase activity was determined by the method of Fritz and Hamrick17 as supported by Desaiah and Ho.18 The reaction mixture contained 135 mM imidazole HCl buffer (pH 7.5), 5 mM MgCl2, 0.05 mM CaCl2, 4 mM ATP, and 30-40 mg protein. The mixture was incubated at 37°C for 30 min and stopped by the addition of 0.1mL of 50% TCA. The inorganic phosphate (Pi) liberated was estimated. Mg2+-ATPase activity was measured in the presence of 0.5 mM EDTA and the value subtracted from ATPase activity was expressed as μ moles of Pi formed/mg protein/hour.
Estimation of inorganic phosphates
Inorganic phosphates were estimated by the method of Fiske and Subba Row.19 To 1.0 mL of the above supernatant, 1.0 mL of ammonium molybdate solution (2.5 g in 100 mL of 10 N H2So4) was added followed by 0.4 mL of ANSA (1-amino 2-naphthol 4-sulphuric acid) and allowed to react for 5 min. The blue color formed was read at 600 nm in a spectrophotometer against a reagent blank. The blank contained 2.0 mL TCA, 1.0 mL ammonium molybdate, and 4.0 mL ANSA. The enzyme activity was expressed as μ moles of inorganic phosphate formed/mg protein/hour.
Protein estimation
Protein content of the selected brain tissue regions was estimated by the method of Lowry et al.20 To 0.5 mL of crude homogenate, 1mL of 10% TCA was added and the samples were centrifuged at 1000 g for 15 min. Then supernatant was discarded and the residue was dissolved in 0.5 mL of 1 N NaoH. To this, 4 mL of alkaline copper reagent was added followed by 0.4 mL of folin-phenol reagent (1:1 folin-phenol reagent: H20). The color was measured at 600 nm in a UV-visible spectrophotometer against blank.
Statistical analysis
All the experiments were carried out in triplicate and expressed as mean ± SD. One-way ANOVA was used to test the significance of differences among four different groups followed by Tukey and Dunnet’s multiple range tests. Statistical analysis was performed using SPSS (version 20; IBM SPSS Inc., Chicago, 1L, USA). The data were regarded as significantly different at p<0.05.
Results
Total ATPases
The results obtained in the present study clearly demonstrated that for 60 days the activity levels of total ATPases in the control rat brain were maximumin in CC (44.65 µmoles of Pi formed/mg protein/hour) followed by HP (40.43 µmoles of Pi formed/mg protein/hour). Conversely, in AD model group, their levels were reduced significantly by -21.87% and -19.05% in CC and HP, respectively (Fig. 1). Interestingly, oral administration of L. plantarum to AD-induced rats (protective group) showed a significant elevation in the activity levels of total ATPases by-3.44% in CC and 1.67% in HP, compared to AD model group.
Fig. 1.
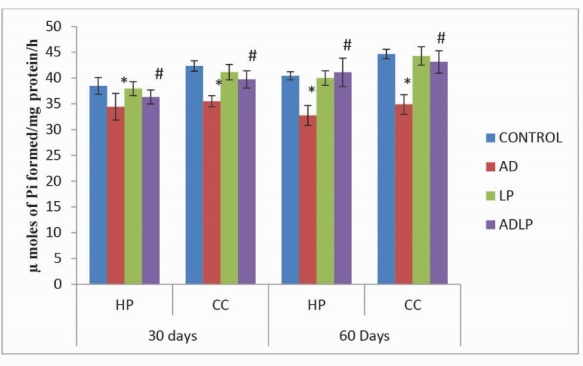
Effect of Lactobacillus plantarum MTCC1325 on the activity levels of total ATPases in hippocampus and cerebral cortex regions of control and experimental groups of rats on 30th and 60th days. *Significant difference at p < 0.05 as compared with the control group. #Significant difference at p < 0.05 as compared with the AD model group. HP - Hippocampus; CC- Cerebral cortex; AD – Alziemer's disease; LP - L. plantarum; ADLP - Alziemers disease + L. plantarum.
Na+, K- ATPase
Similar to total ATPases, comparison of the levels of Na+, K+-ATPases in the CC and HP regions of AD model rat brain showed that they were relatively lower by -15.24% and -17.33%, respectively, compared to the control group (Fig. 2). However, similar to that of the total ATPases, oral simultaneous administration of L. plantarum MTCC1325 to AD-induced rats for 60 days, caused a significant enhancement in the levels of Na+, K+-ATPases (HP: -2.25% and CC: 0.55%) as compared with AD model group, thereby revealing the positive effect of L. plantarum in the restoration of AD-induced effects close to control levels.
Fig. 2.
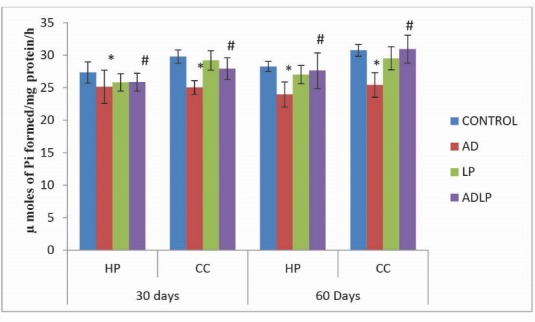
Effect of Lactobacillus plantarum MTCC1325 on the activity levels of Na+/K+- ATPases in hippocampus and cerebral cortex regions of control and experimental groups of rats on 30th and 60th days. *Significant difference at p <0.05 as compared with the control group. # Significant difference at p <0.05 as compared with the AD model group. HP - Hippocampus; CC- Cerebral cortex; AD – Alziemer's disease; LP - L. plantarum; ADLP - Alziemers disease + L. plantarum.
Mg2+- ATPases
As similar to that of Na+, K+-ATPases, the activity levels of Mg2+-ATPases were also significantly reduced in HP (-27.89%) and CC (-31.91%) regions of AD model group rat brains when compared to control (Fig. 3). However, prolonged (60 days) administration of L. plantarum to AD-induced group rats showed a significant increase in the Mg2+-ATPases activity levels (HP: 10.78% and CC: -12.29%) compared to the AD model group.
Fig. 3.
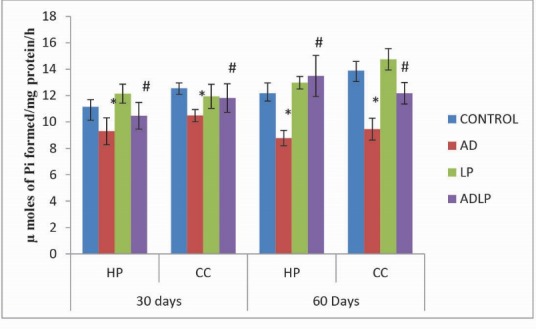
Effect of Lactobacillus plantarum MTCC1325 on the activity levels of Mg2+- ATPases in hippocampus and cerebral cortex regions of control and experimental groups of rats on 30th and 60th days. *Significant difference at p <0.05 as compared with the control group. #Significant difference at p <0.05 as compared with the AD model group. HP - Hippocampus; CC- Cerebral cortex; AD - Alziemers disease; LP - L. plantarum; ADLP - Alziemers disease + L. plantarum.
Ca2+-ATPases
Similar to the other ATPases, the Ca2+-ATPases activity levels were significantly reduced in HP (-20.63%) and CC (-19.37%) regions of AD model rat brain when compared to the control groups (Fig. 4). Coversely, a significant increase in Ca2+-ATPases activity levels (HP:20.90% and CC: 20.09%) were observed on the simultaneous treatment of AD-induced rats with Lactobacillus plantarum MTCC1325 for 60 days.
Fig. 4.
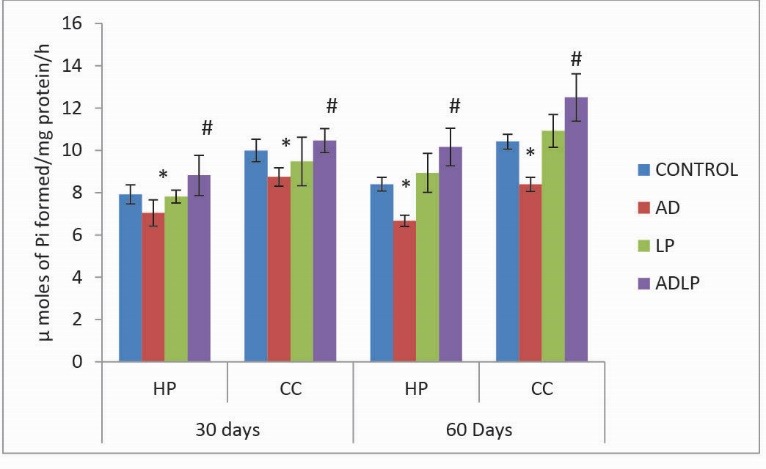
Effect of Lactobacillus plantarum MTCC1325 on the activity levels of Ca2+- ATPases in hippocampus and cerebral cortexregions of control and experimental groups of rats on 30th and 60th days. *Significant difference at p <0.05 as compared with the control group. # Significant difference at p <0.05 as compared with the AD model group. HP - Hippocampus; CC- Cerebral cortex; AD - Alziemers disease; LP - L. plantarum; ADLP - Alziemers disease + L. plantarum.
Discussion
In the present study, we evaluated the efficiency of L. plantarum MTCC1325 on the activity levels of membrane bound transport ATPases including Na+, K+-ATPases, Mg2+-ATPases, and Ca2+-ATPases in the HP and CC regions of D-Galactose-induced Alzheimer’s disease male Wistar rats brain.
From our results, it was obvious that AD induction by the chronic injection of D-Galactose reduced total ATPases activities in the selected regions (HP and CC) of rat brain when compared to the normal control group. This observation derives strong support from earlier reports which proved that long- term exposure of rats to D-Galactose impaired the activities of mitochondrial enzymes, membrane permeability, and ATP production.13,21 In the present experiment, it was also observed that Na+, K+-ATPases were high in protective group (D-galactose + L. plantarum) compared to the normal control group. Further, the remaining ATPases including Mg2+-ATPases and Ca2+-ATPases showed similar levels when AD-induced rats were treated with L. Plantarum for 30 days; but 60 days treatment showed significantly increased activity levels of ATPases in protective group compared to the normal control and AD model groups. An interesting finding in the present study was that oral administration of L. plantarum could effectively reverse the D-Galactose-induced AD effect in all experimental groups of rats. The results clearly demonstrated that AD-induced rats simultaneously treated with L. plantarum showed increasing activity levels of ATPases in CC region followed by HP region of the rat brain compared to AD model group. A very recent report by Rai et al22 showed that the rats fed with fish visceral waste through lactic acid fermentation by P. acidilactici NCIM5368 enhanced the Na+, K+-ATPases in brain. This study lends strong support and reiterates our present observations on all types of ATPases. Furthermore, several clinical trial findings established that treatment of irritable bowel syndrome patients with lactobacillus strain (Lactobacillus plantarum 299v) showed significant improvement from symptoms.23-25
ATP is continuously required by membrane ATPases to maintain ionic homeostasis across the membrane, lipid asymmetry, and cell to cell communication. In general, the Na+, K+-ATPases play an important role in learning and memory, which further establish that an impairment in the function of these membrane ATPases leads to the initiation of AD pathogenesis.26,27 The oxidative stress and dysfunction of redox-related enzymes disrupt energy metabolism in the brain, eventually resulting in the reduction of energy utilization in the AD brain.28,29 Thus, Na+, K+-ATPases play a major role in the augmentation of neuronal pathogenesis in Alzheimer’s disease. Furthermore, it has been described that the stimulators and therapeutics involved in the enhancement of Na+, K+-ATPase activities in AD are considered as potent neuroprotective agents against AD.30
Our present findings are justified on the assumption that the supplementation of rat diet with L. plantarum might cause the enhancement of membrane bound transport ATPases activities which is in turn associated with its antioxidant properties. Further, L. plantarum may produce a variety of beneficial secondary metabolites which are involved in various biochemical pathways of the brain through the enteric nervous system (Gut-brain axis) and thus protect the neurons by stabilizing the structural and functional integrity of the biological membranes through the regulation of ionic concentration gradient.31 However, further research is required on higher mammalian experimental models such as rabbits, owl monkeys, vervet monkeys, squirrel monkeys, and so on to better understand the possible role of Lactobacillus strains in the protection against neurodegenerative diseases.
Conclusion
Based on the results of the present study, it can be concluded that the supplementation with L. plantarum MTCC1325 significantly improved the activities of the membrane transport ATPases system in the selected brain regions of AD-induced group. Further, it was revealed that L. plantarum MTCC1325 protects the neurons by stabilizing the structural and functional integrity of the biological membranes through the regulation of ionic concentration gradient by its antioxidant properties.
Acknowledgements
The First author, Mr. N. Mallikarjuna, is highly thankful to University Grants Commission, New Delhi for its financial support through BSR Fellowship and acknowledges the head of Department of Zoology, S.V University, Tirupati, Andhra Pradesh, India for providing the laboratory facilities to carry out this research.
Competing interests
The authors declare no conflict of interest regarding the publication of this paper.
Ethical approval
The research work was carried out and the experimental rats were maintained according to the guidelines of Institutional Animal Ethical Committee (Resolution No. 34/20122013/(i)/a/ CPCSEA/IAEC/ SVU/KY dt.01.07.2012).
Research Highlights
What is current knowledge?
√ Alzheimer’s disease is a common form of dementia, mostly found in the elder people, above 65 years.
√ Since many several years, various bioactive compounds from medicinal plants, marine algae, neutraceuticals and functional foods have been tested for their therapeutic activities against AD.
√ Nowadays, probiotics and LAB play a curative role in different types of diseases with their beneficial biological activities.
What is new here?
√ The usage of LAB as a potential therapeutic agent is a novel approach to modulate ATPases and subsequently for the treatment of D-Galactose-induced Alzheimer’s disease in rat models, proved biochemically with biostatistical analyses.
√ Our research findings demonstrated that L. plantarum MTCC1325 potentially improves the activities of ATPases in D- Galactose-induced Alzheimer’s rat brain through various biochemical metabolic pathways involved in the bidirectional communication between the gut bacteria and brain.
References
- 1.Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM. et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mattson MP. Pathways towards and away from Alzheimer’s disease. Nature. 2004;430:631–639. doi: 10.1038/nature02621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Butterfield AD, Reed T, Newman SF, Sultana R. Roles of amyloid β-peptide-associated oxidative stress and brain protein modifications in the pathogenesis of Alzheimer's disease and mild cognitive impairment. Free Radic Biol Med. 2007;43:658–677. doi: 10.1016/j.freeradbiomed.2007.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lei M, Su Y, Hua X, Ding J, Han Q, Hu G. et al. Chronic systemic injection of D-galactose impairs the septohippocampal cholinergic system in rats. Neuroreport. 2008;19:1611–1615. doi: 10.1097/WNR.0b013e3283136a1f. [DOI] [PubMed] [Google Scholar]
- 5. Georgina Rodríguez de Lores Arnaiz1 , María Graciela LopezOrdieres. Brain Na+, K+-ATPase Activity In Aging and Disease. Int J Biomed Sci. 2014;10:85–102. [PMC free article] [PubMed] [Google Scholar]
- 6. Victor M. Vitvitskya, Sanjay K. Garga,1, Richard F. Keepb, Roger L. Albinc,d, and Ruma Banerjee. Na+ and K+ ion imbalances in Alzheimer’s disease. Biochim Biophys Acta. 2012;1822(11):1671-81. doi: 10.1016/j.bbadis.2012.07.004. [DOI] [PMC free article] [PubMed]
- 7.Bernardeau M, Vernoux JP, Henri-Dubernet S, Guéguen M. Safety assessment of dairy microorganisms: The Lactobacillus genus. Int J Food Microbiol. 2008;126(3):278–85. doi: 10.1016/j.ijfoodmicro.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 8.Masood MI, Qadir MI, Shirazi JH, Khan IU. Beneficial effects of lactic acid bacteria on human beings. Crit Rev Microbiol. 2011;37(1):91–8. doi: 10.3109/1040841X.2010.536522. [DOI] [PubMed] [Google Scholar]
- 9.Hemarajata P, Versalovic J. Effects of probiotics on gut microbiota: mechanisms of intestinal immunomodulation and neuromodulation. Therap Adv Gastroenterol. 2013;6(1):39–51. doi: 10.1177/1756283X12459294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Islam MS, Choi H. Antidiabetic effect of Korean traditional Baechu (Chinese cabbage) kimchi in a type 2 diabetes model of rats. J Med Food. 2009;12:292–7. doi: 10.1089/jmf.2008.0181. [DOI] [PubMed] [Google Scholar]
- 11.Jung IH, Jung MA, Kim EJ, Hem MJ, Kim DH. Lactobacillus pentosus var plantarum C29 protects scopolamine induced memory deficit in mice. J Appl Microbiol. 2012;113:1498–506. doi: 10.1111/j.1365-2672.2012.05437. [DOI] [PubMed] [Google Scholar]
- 12.Woo JY, Gu W, Kim KA, Jang SE, Han M, Kim DH. Lactobacillus pentosus var plantarum C29 ameliorates memory impairment and inflammation in a D-galactose-induced accelerated aging mouse model. Anaerobe. 2014;27:22–6. doi: 10.1016/j.anaerobe.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 13.Peng X, Meng J, Chi T, Liu P, Man C, Liu S. et al. Lactobacillus plantarum NDC 75017 alleviates the learning and memory ability in aging rats by reducing mitochindraildysfunction. Exp Ther Med. 2014;8:1841–6. doi: 10.3892/etm.2014.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hanish Singh JC, Alagarsamy V, Diwan PV, Sathesh Kumar S, Nisha JC, Narsimha Reddy Y. Neuroprotective effect of Alpiniagalanga (L) fractions on Abeta(25-35) induced amnesia in mice. J Ethnopharmacol. 2011;138(1):85–91. doi: 10.1016/jep.2011.08.048. [DOI] [PubMed] [Google Scholar]
- 15.Stephenson M, Rowatt E, Harrison K. The production of acetylcholine by a strain of Lactobacillus plantarum. J Gen Microbiol. 1947;1:279–98. doi: 10.1099/00221287-1-3-279. [DOI] [PubMed] [Google Scholar]
- 16.Tirri R, Lagrspetz KY, Kohomen J. Temperature dependence of the ATPase activity in brain homogenates during the postnatal development of rat. Comp Biochem Physiol B. 1973;44(2):473–80. doi: 10.1016/0305-0491(73)90021-7. [DOI] [PubMed] [Google Scholar]
- 17.Fritz DJ, Hamrick ME. Enzymatic analysis of (rat heart) adenosine triphosphatase. Enzymology Acta Biochemistry Catalyst. 1966;9:57–64. [Google Scholar]
- 18.Desaiah D, Ho IK. Effects of acute and continuous morphine administration on catecholamine sensitive ATPase in mouse brain. Pharmacol Exp Ther. 1979;208(1):80–5. [PubMed] [Google Scholar]
- 19.Fiske CH, Subba Row Y. The colorimetric determination of phosphates. J Biol. Chem1925;66:375–400. [Google Scholar]
- 20.Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ. Protein measurement with the folinphenol reagent. J. Biol Chem1951 ;193:265–75. [PubMed] [Google Scholar]
- 21.KalyaniBaiKunte, Yellamma Kuna. Neuroprotective effect of Bacopa Monnieri on memory deficits and ATPase system in Alzheimer’s disease (AD) induced mice. Journal of Scientific and Innovative Research. 2013;2(4):719–735. [Google Scholar]
- 22.Rai AK, Bhaskar N, Baskaran V. Effect of feeding lipids recovered from fish processing waste by lactic acid fermentation and enzymatic hydrolysis on antioxidant and membrane bound enzymes in rats. J Food Sci Technol. 2015;52(6):3701–10. doi: 10.1007/s13197-014-1442-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ducrotte P, Sawant P, Jayanthi V. Clinical trial: Lactobacillus plantarum 299v (DSM 9843) improves symptoms of irritable bowel syndrome. World J Gastroenterol. 2012;18(30):4012–8. doi: 10.3748/wjg.v18.i30.4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nobaek S, Johansson ML, Molin G, Ahrné S, Jeppsson B. Alteration of intestinal microflora is associated with reduction in abdominal bloating and pain in patients with irritable bowel syndrome. Am J Gastroenterol. 2000;95:1231–8. doi: 10.1111/j.1572-0241.2000.02015.x. [DOI] [PubMed] [Google Scholar]
- 25.Niedzielin K, Kordecki H, Birkenfeld B. A controlled, double-blind, randomized study on the efficacy of Lactobacillus plantarum 299V in patients with irritable bowel syndrome. Eur J Gastroenterol Hepatol. 2001;13:1143–7. doi: 10.1097/00042737-200110000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Jovicic ME, Popovic M, Nesic KJ, Popovic N, Pavlovic SJ, Rakic L. Aging, aluminium and basal forebrain lesions modify substrate kinetics of erythrocyte membrane Na,K-ATPase in the rat. J Alzheimers Dis. 2008;14:85–93. [PubMed] [Google Scholar]
- 27.Tsai SJ, Lin CY, Mong MC, Ho MW, Yin MC. s-Ethyl cysteine and s-propyl cysteine alleviate beta-amyloid induced cytotoxicity in nerve growth factor differentiated PC12 cells. J Agric Food Chem. 2010;58(11):7104–8. doi: 10.1021/jf1009318. [DOI] [PubMed] [Google Scholar]
- 28.Messier C, Gagnon M. Glucose regulation and brain aging. J Nutr Health Aging. 2000;4:208–13. [PubMed] [Google Scholar]
- 29.Chakraborty H, Sen P, Sur A. Age related oxidative inactivation of Na+-K+-ATPase in rat brain crude synaptosomes. Exp Gerontol. 2003;38:705–710. doi: 10.1016/s0531-5565(03)00066-4. [DOI] [PubMed] [Google Scholar]
- 30.Zhang LN, Sun YJ, Pan S, Li JX, Qu YE, Li Y. et al. Na+-K+-ATPase, a potent neuroprotective modulator against Alzheimer disease. Fundam Clin Pharmacol. 2013;27:96–103. doi: 10.1111/fcp.12000. [DOI] [PubMed] [Google Scholar]
- 31.Hemarajata P, Versalovic J. Effects of probiotics on gut microbiota: mechanisms of intestinal immunomodulation and neuromodulation. Therap Adv Gastroenterol. 2013;6(1):39–51. doi: 10.1177/1756283X12459294. [DOI] [PMC free article] [PubMed] [Google Scholar]


