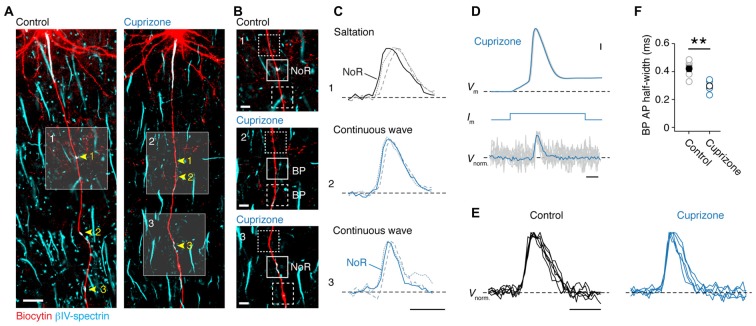
Figure 2.
Loss of saltatory propagation and narrowing of nodal action potentials (APs) in demyelinated axons. (A) Z-projected confocal images of primary layer 5 axons immuno-labeled for (biocytin, red) and βIV-spectrin (cyan). Nodes of Ranvier (noR) in myelinated (left) and BPs in demyelinated (right) axons are highlighted. Scale bar, 20 μm. (B) Zoomed in regions of interest indicated by white squares in (A). Examples of normal noR pattern in control mice (top) and BPs from cuprizone treated mice: lacking βIV-spectrin (middle) and βIV-spectrin enriched (bottom). Scale bar, 5 μm. (C) Top: normalized voltage-sensitive dye (VSD) traces from noR (solid line), preceding internode (dotted line) and following internode (dashed line). Middle and bottom: normalized VSD traces from BP (solid line), more proximal area (dotted line) and more distal area (dashed line). Scale bar, 0.5 ms. (D) Align and overlay of nine example (out of 100) somatic APs and their corresponding BP spikes (VSD recorded at 20 kHz, gray) and the average of all 100 recorded traces (black). Somatic single APs (top) were elicited through brief (3 ms; middle) square current pulse. BP, branch point. Scale bar, 0.5 ms; 10 mV. (E) Normalized VSD traces from control (5 noR; n = 5 cells) and cuprizone-treated mice (6 BPs; n = 5 cells). Scale bar, 0.5 ms. (F) Comparison of BP AP half-widths in control and cuprizone treated mice obtained from VSD data. APs in cuprizone treated mice are significantly narrower (control, n = 5 noR; cuprizone, n = 6 BPs; Mann-Whitney (M-W) test, **P = 0.0087). Individual cells plotted as open circles.