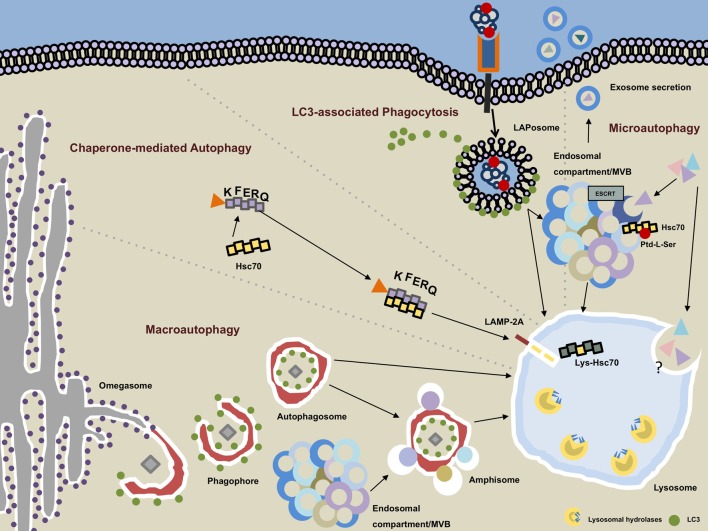
Figure 1.
Autophagy pathways converge in the lysosomal compartment. Macroautophagy (MA): the phagophore emanates most likely from endoplasmic reticulum-derived membrane sources at a PI3P-rich (not depicted) structure called the omegasome. Upon recruitment of microtubule-associated protein 1 light chain 3 (LC3) on the outer and inner leaflet of the forming autophagosome, cytoplasmic cargo is engulfed and LC3 removed from the outer membrane as the autophagosome is closed. The completed vesicles may now either be further matured via step-wise fusion with the endocytic compartment resulting in the generation of amphisomes or immediately fuse with hydrolytic enzymes-containing lysosomes. Chaperone-mediated autophagy: target proteins that contain an exposed KFERQ sequence motif are guided in an hsc70-dependent manner toward lysosome-associated membrane protein type 2A (LAMP-2A), which resides in the lysosomal membrane. Upon unfolding of the target protein, LAMP-2A multimers together with lysosomal hsc70 facilitate the transport into the lysosomal lumen. LC3-associated phagocytosis: ligation of an appropriate receptor (e.g., TLR2, Dectin-1, TIM4, etc.) leads to receptor-mediated phagocytosis recruitment and binding of LC3 to the outer membrane of the LAPosome. By analogy with the MA pathway, completed LAPosomes may either fuse with other endocytic vesicles or directly merge with lysosomes. Microautophagy: cytoplasmic cargo can be directly targeted to endosomal compartments/multivesicular bodies (MVB) in an hsc70-, phosphatidylserine (Ptd-L-Ser)-, and endosomal sorting complexes required for transport (ESCRT)-dependent manner. Exosomes containing cytoplasmic material may emanate from the MVB and be secreted into the extracellular space. The molecular events and regulatory processes orchestrating the direct invagination of cytoplasmic constituents into lysosomes remain largely unbeknownst.