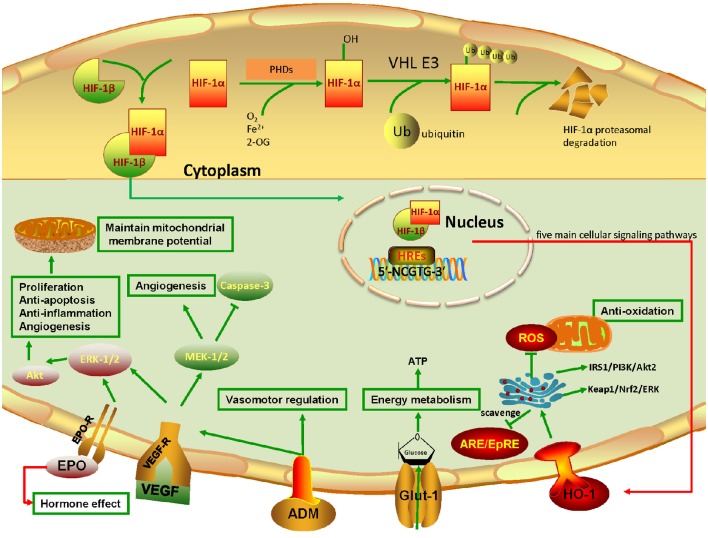
Figure 2.
Pathways of HIF-1α and its target genes involved in retinal neuroprotection. The upper panel in yellow background is the schematic representation of HIF-1α degradation under normoxia. Note that the undegraded HIF-1α binds with HIF-1β to form the HIF-1α/β complex. The complex binds to HIF-responsive elements (HREs) in promoters that contain the sequence motif 5′-NCGTG-3′ and triggers transcription of more than 100 downstream genes. VHL: von Hippel–Lindau tumor suppressor protein (E3 ubiquitin protein ligase). The lower panel in pale blue color represents the HIF-1α target genes and their acting pathways involved in retinal neuroprotection under hypoxia. Note that five main cellular signaling pathways mediating the effect of neuroprotection are highlighted. Firstly, EPO binds to EPO-R to promote ERK-1/2 signaling, and then activate the Akt pathway, resulting in cell proliferation, anti-apoptosis, anti-inflammation, and angiogenesis. Also, it can maintain mitochondrial membrane potential to prevent mitochondrial alteration. In particular, EPO can be pumped outside the cytoplasm, which leads to the autocrine and paracrine effects that further exert retinal neuroprotection. Secondly, VEGF, which binds to VEGF-R, can achieve the same effects as EPO through activating ERK-1/2 signaling. Simultaneously, it enhances the MEK-1/2 pathway, promoting angiogenesis and inhibiting caspase-3 to constrain cell death. Thirdly, the secreted multifunctional peptide ADM mainly plays roles in vasomotor regulation, and acts together with VEGF to promote angiogenesis. Fourthly, Glut-1 transports glucose to the cytoplasm, allowing normal metabolic activity. Fifthly, HO-1 is degraded by ARE/EpRE elements. While under hypoxia, HO-1 blunts reactive oxygen species (ROS) production and the toxic effect on mitochondria. More importantly, HO-1 retards retinal injury through the IRS1/PI3K/Akt2 and Keap1/Nrf2 pathways, which further activate mTOR, upregulate anti-apoptotic proteins, and eliminate ROS.