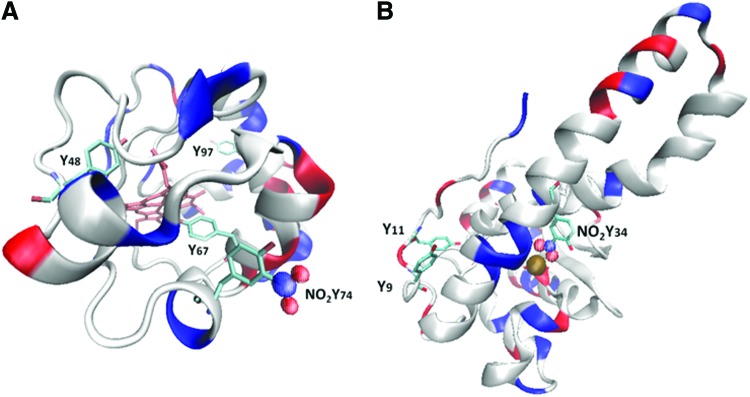
FIG. 3.
Three-dimensional structures of selected regio-specific tyrosine-nitrated proteins. (A) Structure of horse cytochrome c showing nitrated Tyr 74 (modified from pdb 1HRC). Both Tyr 74 and Tyr 97 are prone to nitration by peroxynitrite (14). The solvent buried Tyr 67 adjacent to the heme moiety is prone to nitration by peroxidase-dependent mechanisms (23, 24). Tyr 48, although not readily nitrated, has been found to be phosphorylated (123). The four described tyrosine residues in horse cytochrome c are highly conserved, whereas another tyrosine (Tyr 46) is present in human cytochrome c. (B) Structure of human manganese superoxide dismutase (hMnSOD) showing nitrated Tyr34 (pdb 2ADP). Tyr 34 is part of the superoxide radical entrance channel and active site, and its nitration leads to enzyme inactivation (67, 119). Also shown are surface-exposed Tyr 9 and Tyr 11, which undergo nitration by peroxidase-dependent mechanisms (112). hMnSOD also has Tyr at positions 45, 165, 166, 169, 176, and 193 that are not shown for the sake of clarity in the diagram. The structures were drawn using VMD Software (50). VMD was developed by the Theoretical and Computational Biophysics Group in the Beckman Institute for Advanced Science and Technology at the University of Illinois at Urbana-Champaign. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars