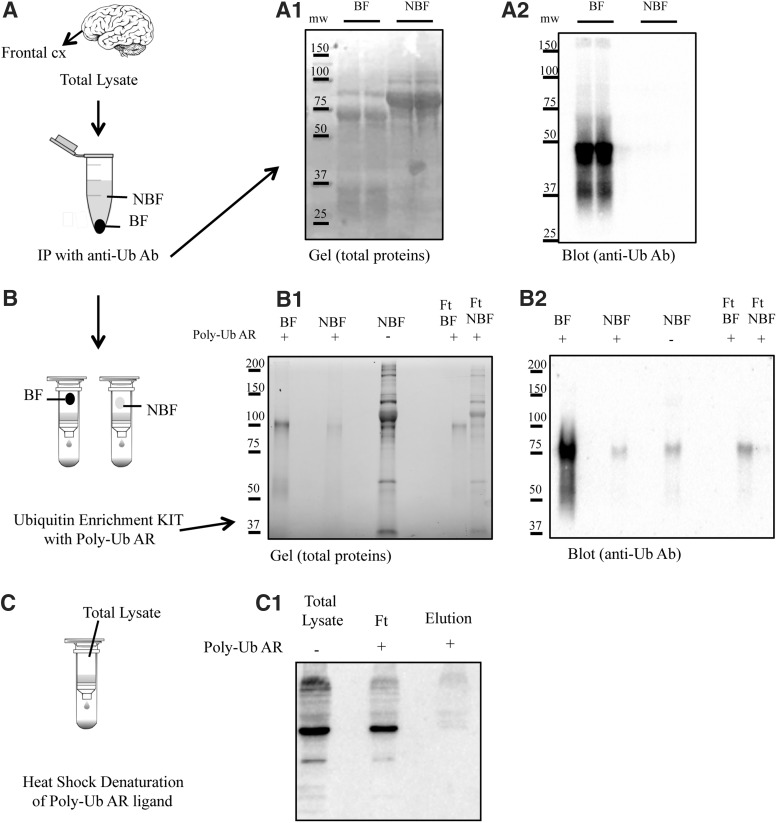
FIG. 1.
Isolation of endogenously polyubiquitinated protein complexes by use of the ubiquitin enrichment kit. A workflow for isolating endogenously ubiquitinylated protein complexes for proteomic analysis is shown. (A) Ubiquitinylated proteins were isolated from brain homogenates by IP. IP fraction (BF) and the supernatant (NBF) were loaded on an SDS-PAGE gel and stained for total protein expression (A1) and subsequently blotted on nitrocellulose membrane and stained with a polyclonal anti-Ub antibody (anti-Ub Ab) to determine enrichment efficiency (A2). (B) The IP fractions (BF and NBF) were further processed with the enrichment kit (Thermo) containing the polyubiquitin affinity resin (Poly-Ub AR). BF, NBF, and FT were loaded in SDS page (B1) and blotted with antiubiquitin antibody (B2). (C) Heat denaturation of high-affinity resin using high temperature (60°) was used to destroy the bond between resin and poly-Ub antibody. Poly-Ub was detected by performing a Western blot (agarose-only control; C1). BF, bound fraction; FT, flow-through; IP, immunoprecipitation; NBF, nonbound fraction; Poly-Ub, polymeric Ub; SDS-PAGE, sodium dodecyl sulfate–polyacrylamide gel electrophoresis; Ub, ubiquitin.