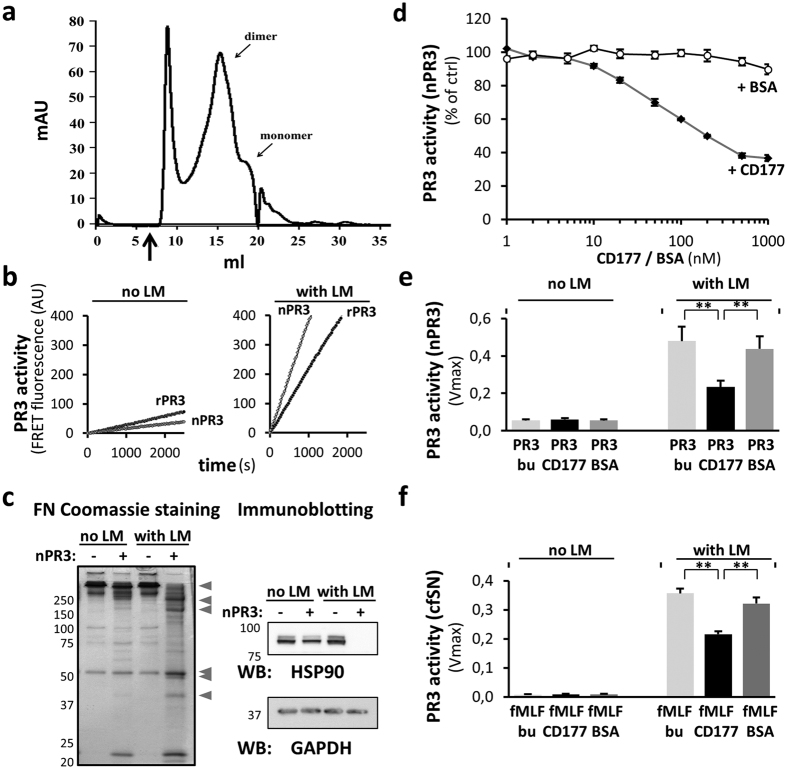
Figure 4. PR3 solubility and activity are increased by detergent.
(a) S200 size exclusion chromatogram of PR3 in the presence of 0.02% lauryl maltoside (LM). Apparent monomer and dimer species are indicated; the arrow marks the void volume of the column (b) Enzymatic activity FRET assays with 1 nM rPR3 and nPR3 in the absence (no) or presence (with) of 0.02% LM. (c) nPR3 incubated with fibronectin (FN) or HUVECs protein extract. 16 μg/lane FN was loaded onto a 10% SDS gel and Coomassie stained. Note that several additional FN cleavage products (arrowheads) are seen in the presence of LM. Increased cleavage was also observed by immunoblotting for HSP90 when nPR3 digested HUVECs protein in the presence of LM. (d) The effect of rCD177 on the enzymatic nPR3 activity was studied by FRET assay. A dose-dependent activity reduction was observed by incubating 0.5 nM nPR3 with increasing rCD177 concentrations from 1 to 1,000 nM in the presence of 0.02% LM (n = 3). BSA served as control protein. (e) The effect of rCD177 on nPR3 activity in the absence and presence of 0.02% LM was studied by FRET assay. 0.5 nM nPR3 was incubated with 50 nM rCD177 or BSA, respectively (n = 5). bu indicates buffer alone control. (f) The effect of 50 nM rCD177 on PR3 activity in cell-free supernatants (cfSN) from neutrophils stimulated with 10−7 M fMLF was measured in the absence and presence of 0.02% LM by FRET assay (n = 5). ** is p < 0.01.