Abstract
Objective. Tumor necrosis factor (TNF) increases circulating osteoclast (OC) precursors numbers by promoting their proliferation and differentiation. The aim of this study was to assess the effect of TNF inhibitors (TNFi) on the differentiation and activity of OC in rheumatoid arthritis (RA) patients. Methods. Seventeen RA patients treated with TNFi were analyzed at baseline and after a minimum follow-up period of 6 months. Blood samples were collected to assess receptor activator of nuclear factor kappa-B ligand (RANKL) surface expression on circulating leukocytes and frequency and phenotype of monocyte subpopulations. Quantification of serum levels of bone turnover markers, in vitro OC differentiation assays, and qRT-PCR for OC specific genes was performed. Results. After TNFi therapy, patients had reduced RANKL surface expression in B-lymphocytes and the frequency of circulating classical CD14brightCD16− monocytes was decreased. Serum levels of sRANKL, sRANKL/OPG ratio, and CTX-I were reduced in RA patients after TNFi treatment. Moreover, after exposure to TNFi, osteoclast differentiation and activity were decreased, as well as the expression of TRAF6 and cathepsin K. Conclusion. We propose that TNFi arrests bone loss and erosion, through two pathways: direct reduction of osteoclast precursor numbers and inhibition of intracellular signaling pathways acting through TRAF6.
1. Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory disease characterized by systemic inflammation, bone erosion, and secondary osteoporosis [1].
The immune and skeletal systems have several regulatory factors in common and immune system cells have a profound influence on bone metabolism, particularly in the context of chronic inflammatory diseases. Receptor activator of nuclear factor-κB ligand is present on osteoblasts' surface but is also expressed by activated immune cells, both in its membrane form and as a soluble molecule [2]. Tumor necrosis factor (TNF) increases the trafficking of immune system cells that efflux from bone marrow and peripheral blood into secondary lymphatic organs and sites of inflammation and is abundantly found in rheumatoid joints [3]. TNF, together with other cytokines, acts synergistically with the RANK-RANKL system [3, 4], further enhancing osteoclast (OC) differentiation from its circulatory precursors (monocytes) and contributing to bone resorption [2, 5]. It also increases the number of circulating OC precursors and the proinflammatory cytokine levels in RA patients. These effects are achieved with low levels of circulating TNF and thus TNF quantification is frequently unreliable in RA patients [6–8]. Of interest, TNF inhibitors (TNFi) have a beneficial effect in delaying radiographic damage in RA patients, even in the absence of clinical improvement, suggesting a specific effect of TNF inhibition, independent of inflammation control [9]. Whether this specific effect of TNFi in preventing bone damage in fact occurs independently of the overall inflammatory burden and whether it occurs because of reduced OC number and/or function are still unclear.
Our hypothesis was that, in RA patients, TNFi decrease the OC circulating precursors' differentiation potential and activity. Thus, the aim of this study was to assess the effect of TNFi in the differentiation and activity of OC precursors in a cohort of RA patients, evaluating also the correlation between clinical manifestations of inflammation and OC related parameters.
2. Patients and Methods
2.1. Patients
Patients with RA fulfilling the 2010 American College of Rheumatology/European League Against Rheumatism criteria [10] were recruited from the Rheumatology Department, Hospital de Santa Maria, Lisbon Academic Medical Centre, Portugal. All RA patients included were TNFi naïve and were followed up during a minimum of 6 months after starting TNFi therapy. Information regarding patients' demographics, duration of symptoms, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), tender and swollen joints counts, presence of erosion, presence of rheumatoid factor (RF), and presence of anticitrullinated protein antibodies (ACPA) was collected. Disease activity score (DAS28-CRP) was evaluated, as well as the Health Assessment Questionnaire (HAQ) [11].
Heparinized blood and serum samples were analyzed in baseline and follow-up samples after TNFi treatment approximately 6 months later. Whole blood samples were taken for flow cytometry and for isolation of peripheral blood mononuclear cells (PBMCs). Samples were stored at the Biobanco-IMM, Lisbon Academic Medical Center, Lisbon, Portugal. Patients were managed with the standard practice and all participants gave their informed consent. The study was approved by the local ethics committee and was conducted in accordance with the Declaration of Helsinki as amended in Brazil (2013).
2.2. Flow Cytometry
Identification of B- and T-cells and granulocytes in peripheral blood, RANKL surface expression, and immunophenotyping of monocytes in the PBMC samples were performed using matched combinations of anti-human murine mAbs as previously described [12]. Heparinized whole blood was used for flow cytometry and absolute cell counts were calculated from differential leukocyte count determined for all participants. Mononuclear cells were isolated from freshly drawn peripheral blood using density gradient centrifugation with Histopaque®-1077 (Sigma-Aldrich). Subpopulations of monocytes were identified based on the surface expression of CD14 and CD16 [13]. Median fluorescence intensity (MFI) was calculated based only on positive cells as determined by isotype control gating. FlowJo software (Tree Star, Stanford University) was used for analyzing flow cytometry data.
2.3. Bone Turnover Markers and Bone Metabolism Proteins Detection in the Serum
Carboxyterminal type I collagen cross links (CTX-I) for bone degradation products, human type I procollagen amino terminal propeptide (P1NP, Sunred Biological Technology) for bone formation, sclerostin (SOST), osteoprotegerin (OPG), Dickkopf-related protein-1 (DKK1), and soluble RANKL (ampli-sRANKL, Biomedica Gruppe) were analyzed with enzyme-linked immunosorbent assay [14] in serum samples according to the manufacturer's instructions.
2.4. PBMC Isolation and Cell Culture
PBMCs were isolated by density gradient centrifugation and plated in 96-well culture plates at a density of 7.0 × 105 cells/well as described previously [12]. PBMCs were left overnight for OC precursors to adhere on bone slices and were further cultured for 21 days with macrophage-colony stimulating factor (M-CSF, 25 ng/mL, Peprotech), sRANKL (50 ng/mL, Peprotech), dexamethasone (10 nM, Sigma-Aldrich), and transforming growth factor-β (TGF-β, 2.5 ng/mL, R&D Systems), as described by our group [12]. Adherent cells at day 1 and cells cultured on bone slices for 7, 14, and 21 days [15] were used for functional assays and gene expression.
2.5. Functional Assays
OCs were stained for tartrate-resistant acid phosphatase (TRAP) at days 7, 14, and 21 using the Acid Phosphate Leukocyte Kit (Sigma-Aldrich) according to the manufacturer's instructions. Multinuclear cells containing three or more nuclei [16, 17] were counted as TRAP positive OCs. After visualization, cells were removed from bone slices using sodium hypochlorite and stained with 0.1% toluidine blue for the measurement of resorbed area at days 7, 14, and 21 of culture [18]. Bone slices were photographed in an area of 1.25 mm2 with a bright field microscope (Leica DM2500, Leica). The number of TRAP stained OCs was counted at each time point and resorption pits were traced using ImageJ software (NIH, Bethesda, MD). The resorbed area was expressed in % of total area.
2.6. Gene Expression
RNA was extracted from cells cultured over bone slices at days 1, 7, 14, and 21 of culture using NZYol (NZYTech) and complementary (c)DNA was synthesized as described previously [12]. Genes that encode osteoclast proteins such as RANK, TNF-receptor associated factor-6 (TRAF6), Fos-related antigen-2 (FRA-2), a subunit of H+-dependent ATPase (ATP6V0D2), TRAP, and cathepsin K (CTSK) were studied by real-time quantitative PCR (RT-qPCR) using the DyNAmo™ Flash SYBR Green qPCR Kit (Thermo Scientific). Primers (Suppl. Table 1 in Supplementary Material available online at https://doi.org/10.1155/2017/2690402) were designed using the primer-BLAST software [19]. The results were normalized with the housekeeping gene ribosomal RNA 18s and the standard curve method was used to determine the efficiency of qPCR as described previously [20, 21].
2.7. Statistical Analysis
Statistical analysis was performed with SPSS Statistics 17.0 (IBM) and GraphPad Prism 5 (GraphPad Software Inc.). Categorical variables were expressed as frequencies and comparisons were tested using chi-square test. Continuous variables were expressed by median and interquartile range. Spearman's correlations were performed between the analyzed parameters and clinical variables (ESR, CRP, tender and swollen joint count, and DAS28). Baseline and follow-up values of each sample were compared using Wilcoxon's matched-pairs signed-rank test or paired t-test according to normal distribution. p value less than 0.05 was considered significant.
3. Results
3.1. Patient Background
Seventeen RA patients, evaluated before and after starting TNFi therapy, were included in this study. All patients were receiving methotrexate (10–20 mg weekly), 15 of whom were also under low dose prednisolone and 2 were additionally under bisphosphonates. These therapies had been introduced more than 6 months before TNFi was started and were stable over the study period. Patients were treated with one of four TNFi: one of the monoclonal antibodies (adalimumab, golimumab, or infliximab; 41%) or etanercept (59%). A blood sample was obtained before the start of TNFi and after at least 6 months of treatment. Thirteen patients (76%) were good responders to TNFi and 4 (24%) were moderate responders according to the EULAR response criteria [22]. Joint counts, ESR, CRP, DAS28, and HAQ were significantly decreased after TNFi therapy. The clinical and demographic characteristics of patients both at baseline and at follow-up are described in Table 1.
Table 1.
Baseline and follow-up characteristics of patients.
| RA patients (n = 17) | p-value | |||
|---|---|---|---|---|
| Baseline | Follow-up | |||
| Age (years) | 50 [38–63] | — | ||
| % Females | 71% | — | ||
| Symptoms duration (years) | 6 [3.5–9.5] | — | ||
| Rheumatoid factor (% positive) | 71 | — | ||
| ACPA (% positive) | 53 | — | ||
| Erosive (% y) | 59 | — | ||
| Treatment with NSAIDs (% y) | 47 | — | ||
| Treatment with DMARD (% y) | 100 | — | ||
| DMARD duration (months) | 15 [3–51] | — | ||
| ESR (mm/h) | 28 [18–48] | 21 [13–26] | 0.0257 | |
| CRP (mg/dl) | 1.4 [0.7–2.0] | 0.3 [0.04–0.8] | 0.0018 | |
| Tender joint count | 9 [4–14] | 0 [0–2] | 0.0005 | |
| Swollen joint count | 7 [4–9] | 0 [0-0] | 0.0005 | |
| DAS28-CRP | 5.6 [5.2–6.3] | 2.9 [2.2–3.5] | <0.0001 | |
| HAQ | 1.7 [0.8–2.0] | 0.1 [0.0–1.0] | 0.0059 | |
| TNFi duration (months) | — | 6 [6–12] | — |
Data is represented as median [Interquartile range] unless stated otherwise; p-value < 0.05 is considered significant; ACPA - anti-citrullinated protein antibodies; CRP – C-reactive protein; DAS – disease activity score; DMARDs – disease modifying antirheumatic drugs; ESR – erythrocyte sedimentation rate; HAQ - Health assessment questionnaire; NSAIDs - non-steroidal anti-inflammatory drugs; RA – rheumatoid arthritis; TNFi – tumor necrosis factor inhibitors; y – yes.
3.2. TNFi Treatment in RA Patients Decreases the Frequency of Circulating Osteoclast Precursors
After TNFi treatment, the frequency of the classical monocyte subpopulation (CD14brightCD16−) was decreased (p = 0.0065; Table 2) and that of the nonclassical subpopulation (CD14dimCD16+) was increased (p = 0.0005) [13]. No differences were identified in either CD51/CD61 (αvβ3 integrin) or RANK surface expression. After statistical correction for multiple comparisons, only the increase in the nonclassical subpopulation remained significant.
Table 2.
Monocyte subpopulation frequency and osteoclastogenic marker expression.
| Baseline | Follow-up | p value | |
|---|---|---|---|
| Classic (%)a | 88 [82–89] | 78 [70–83] | 0.0065∗∗ |
| Classic CD51/CD61 MFI | 130 [119–148] | 125 [111–137] | 0.4258 |
| Classic RANK MFI | 133 [116–160] | 122 [100–135] | 0.1849 |
|
| |||
| Intermediate (%)a | 4.4 [2.4–5.6] | 4.0 [2.1–7.1] | 0.6013 |
| Intermediate CD51/CD61 MFI | 222 [139–400] | 193 [146–240] | 0.8203 |
| Intermediate RANK MFI | 197 [117–361] | 188 [120–272] | 0.9102 |
|
| |||
| Nonclassic (%)a | 5.7 [4.1–11] | 14 [11.5–18.1] | 0.0005∗∗∗,† |
| Nonclassic CD51/CD61 MFI | 192 [80–290] | 142 [127–167] | 0.5703 |
| Nonclassic RANK MFI | 139 [122–157] | 138 [126–146] | 1.0000 |
Flow cytometry results are shown as median and interquartile range; agated on the monocyte subpopulation from peripheral blood mononuclear cells. RANK: receptor activator of nuclear factor-κB; MFI: median fluorescence intensity (arbitrary units); ∗∗p < 0.01, ∗∗∗p < 0.001. †Remained significant after correction for multiple comparisons.
RANKL surface staining was performed in CD66b+ neutrophils, CD3+ T-cells, and CD19+ B-cells (Table 3). No difference was found in the total number of circulating neutrophils and T- or B-cells after therapy. Although the frequency of RANKL+ neutrophils or T-cells was not significantly different after treatment, both frequency and absolute number of RANKL+ B-cells were higher after treatment (p = 0.0088 and 0.0029, resp.). However, B-cell RANKL surface expression was significantly decreased after treatment (p = 0.0401). When statistically corrected for multiple comparisons, the increase in RANKL+ B-cells remained significant.
Table 3.
Whole blood cell distribution and RANKL expression.
| Baseline | Follow-up | p value | |
|---|---|---|---|
| Neutrophils (%)a | 82 [71–91] | 90 [84–091] | 0.2662 |
| Neutrophils (×108 cells/L) | 12.7 [8.0–15.6] | 9.6 [8.4–12.9] | 0.2642 |
| RANKL+ neutrophils (%)b | 22 [3–41] | 53 [21–77] | 0.0856 |
| RANKL+ neutrophils (×108 cells/L) | 1.5 [0.3–4.3] | 5.9 [1.8–7.1] | 0.1475 |
| Neutrophil RANKL MFI | 33.2 [25.5–44.9] | 24.1 [21.7–28] | 0.0830 |
|
| |||
| T-cells (%)c | 62 [58–74] | 68 [52–72] | 0.5265 |
| T-cells (×108 cells/L) | 4.2 [2.4–5.2] | 3.4 [2.4–11.7] | 0.4131 |
| RANKL+ T-cells (%)b | 6.2 [0.8–24] | 6.7 [4.6–15.7] | 0.8984 |
| RANKL+ T-cells (×108 cells/L) | 0.30 [0.03–1.03] | 0.20 [0.16–0.69] | 0.7646 |
| T-cell RANKL MFI | 49 [41–55] | 32 [25–53] | 0.2061 |
|
| |||
| B-cells (%)c | 7.3 [4.8–14] | 9.2 [4.9–15.0] | 0.7364 |
| B-cells (×108 cells/L) | 0.40 [0.18–0.94] | 0.44 [0.23–1.51] | 0.9658 |
| RANKL+ B-cells (%) | 4.7 [2.0–6.7] | 14 [3–28] | 0.0088∗∗ |
| RANKL+ B-cells (×108 cells/L)b | 0.02 [0.01–0.06] | 0.06 [0.02–1.22] | 0.0029∗∗,† |
| B-cell RANKL MFI | 48 [38–80] | 30 [25–63] | 0.0401∗ |
Flow cytometry results are shown as median and interquartile range; agated on granulocytes from whole blood; bgated on the correspondent parent gate (neutrophil, T- or B-cell); cgated on the nongranulocyte cells from whole blood (also called the “monolymph” gate). RANKL: receptor activator of NF-κβ ligand; MFI: median fluorescence intensity (arbitrary units); ∗p < 0.05, ∗∗p < 0.01. †Remained significant after correction for multiple comparisons.
3.3. The sRANKL/OPG Ratio and CTX-I Circulating Levels Are Reduced in RA Patients after TNFi Treatment
Circulating levels of sRANKL were significantly decreased after TNFi (p = 0.0085; Table 4), leading to decreased sRANKL/OPG ratio (p = 0.0031). We found no differences in the circulating levels of DKK1 or SOST. CTX-I and P1NP levels were lower in patients at 6 months of follow-up, when compared to patients at baseline (p = 0.0005 and 0.0252, resp.), and no difference was found in the CTX-I/P1NP ratio. After correcting for multiple comparisons, the differences in sRANKL/OPG and CTX-I after treatment remained significant.
Table 4.
Serum levels of bone turnover markers and bone metabolism proteins.
| Baseline | Follow-up | p value | |
|---|---|---|---|
| sRANKL (pmol/L) | 0.32 [0.21–0.67] | 0.18 [0.11–0.35] | 0.0085∗∗ |
| OPG (pmol/L) | 4.34 [2.60–5.82] | 4.22 [3.05–5.08] | 0.7990 |
| sRANKL/OPG | 0.08 [0.04–0.17] | 0.05 [0.03–0.07] | 0.0031∗∗,† |
| DKK1 (pmol/L) | 25.5 [18.1–43.3] | 26.4 [21.9–31.7] | 1.000 |
| Sclerostin (pmol/L) | 25.2 [16.94–33.8] | 25.2 [19.2–29.3] | 0.8577 |
| CTX-I (ng/mL) | 194.6 [176.6–430.7] | 163.6 [152.1–173.9] | 0.0005∗∗∗,† |
| P1NP (ng/mL) | 55.7 [46.3–61.3] | 45.8 [39.6–48.9] | 0.0252∗ |
| CTX/P1NP | 3.36 [3.09–3.82] | 3.71 [3.34–4.30] | 0.5590 |
Enzyme-linked immunosorbent assay results are shown as median and interquartile range. sRANKL: soluble receptor activator of NF-κβ ligand; OPG: osteoprotegerin; DKK1: Dickkopf-related protein-1: CTX: carboxyterminal telopeptide of type I collagen; P1NP: total procollagen type 1 N-terminal propeptide; ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001. †Remained significant after correction for multiple comparisons.
3.4. Osteoclast Differentiation and Activity in RA Patients Are Decreased after TNFi Treatment due to Decreased TNF Intracellular Signaling and Cathepsin K Expression
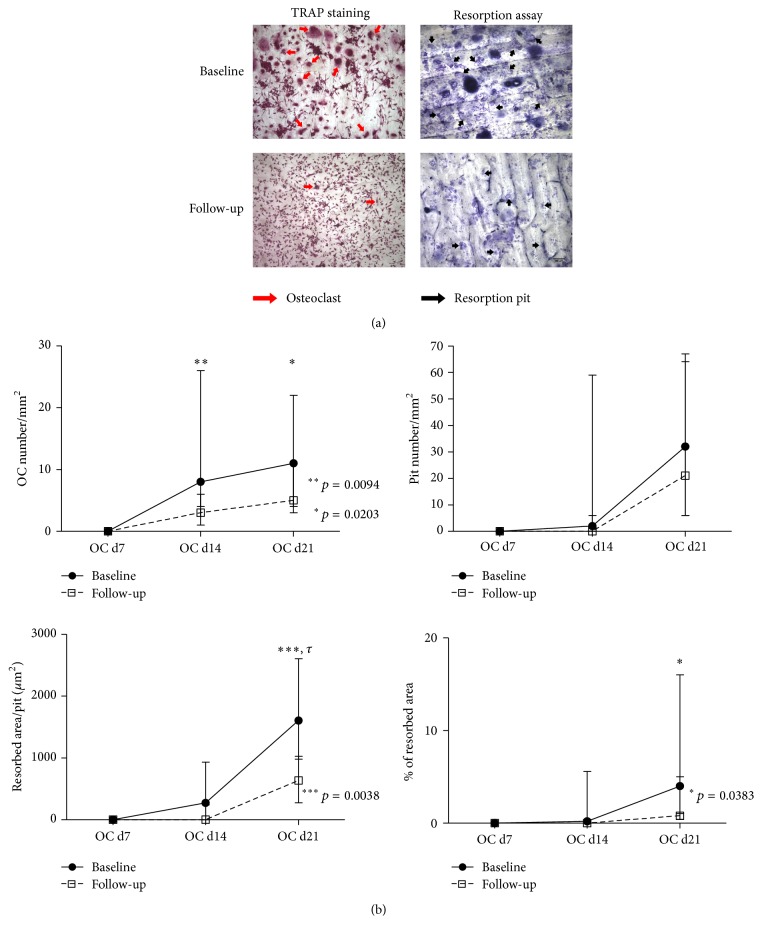
Under stimulating conditions, adhering precursors from patients treated with TNFi formed fewer OCs than adhering precursors from patients at baseline (p = 0.0094 at culture day 14, p = 0.0203 at culture day 21; Figure 1).
Figure 1.
Functional assays of in vitro differentiated OC. (a) Representative images, at culture day 21, of adhering precursors stimulated with M-CSF, RANKL, dexamethasone, and TGF-β stained for TRAP, where the pit assay was performed. (b) OC number increased throughout time and, at culture days 14 and 21, patients at follow-up had significantly fewer osteoclasts than at baseline (p = 0.0094 and 0.0203, resp.). No differences were found in the number of resorption pits/mm2; patients at follow-up had significantly smaller pits at culture day 21 (resorbed area/pit, p = 0.0038) and significantly less resorbed area at culture day 21, when compared to their baseline (p = 0.0383). Dots represent median counts for each group at each time point and bars represent interquartile range. d: day; OC: osteoclast. Scale bars: 100 μm; red arrows: osteoclasts; black arrows: resorption pits. τ: remained significant after adjusting for multiple comparisons.
Although the number of resorption pits was not significantly different before and after treatment, the area resorbed per pit was significantly reduced in cultures from patients at follow-up at culture day 21 (p = 0.0038), which resulted in significantly decreased total resorbed area (p = 0.0383). After statistical correction for multiple comparisons, only the differences in OC number at day 14 and the resorbed area per pit at day 21 remained significant.
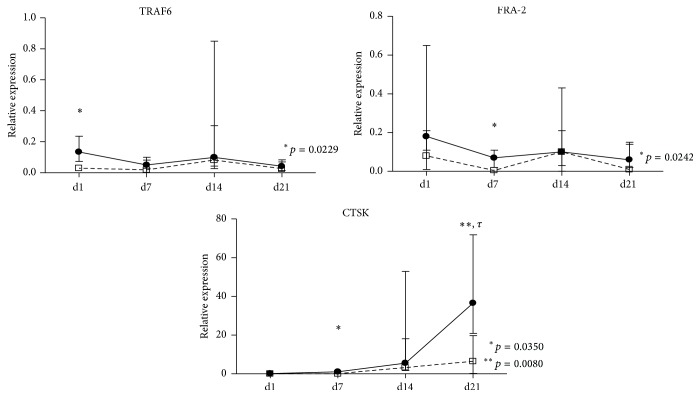
Gene expression by RT-qPCR was performed for OC genes that are known to be important during the adhering precursors' differentiation and OC activity. At culture day 1, TRAF6 expression in patients at follow-up was significantly lower than in patients at baseline (p = 0.0229; Figure 2). At culture day 7, expression of both FRA-2 and CTSK was significantly decreased after TNFi treatment (p = 0.0242 and 0.0350, resp.). No differences were found in any of the studied genes at culture day 14, but at culture day 21 there was a significant decrease in CTSK expression in the differentiated OC from patients after treatment. This difference in CTSK expression remained significant after multiple comparisons adjustment.
Figure 2.
Gene expression profile of stimulated adhering precursors in culture for 21 days. At day 1, TRAF6 expression in patients at follow-up was significantly reduced (p = 0.0229). At day 7, both FRA-2 and CTSK expressions were significantly decreased (p = 0.0242 and 0.035, resp.). At day 21, patients at follow-up had significantly reduced expression when compared to patients at baseline (p = 0.008). Gene expression shown as a ratio to housekeeping expression (2(−ΔCT)/2(−ΔCT)). Dots in graphs represent median gene expression for each group at each time point and lines represent interquartile range [25–75]. d: day; TRAF6: gene encoding tumor necrosis factor receptor-associated factor-6; FRA-2: gene encoding Fos-related antigen-2; CTSK: gene encoding cathepsin K. τ: remained significant after adjusting for multiple comparisons.
No differences were found in any of the studied parameters when comparing monoclonal antibodies (adalimumab, infliximab, or golimumab) with the fusion protein etanercept (data not shown). No correlation was found between clinical or laboratorial inflammatory parameters for any of the studied variables.
4. Discussion
With this study, we aimed to test the effect of TNFi in the differentiation and activity of OC precursors in RA patients.
We have shown that RA patients treated with TNFi have reduced frequency of classic monocytes. We also found a decrease in the circulating levels of soluble RANKL and consequently a reduction in the sRANKL/OPG ratio after TNFi treatment. Although no differences in circulating levels of SOST or DKK1 were detected, serum CTX-I and P1NP levels were decreased after TNFi treatment, reflecting decreased bone turnover in these patients. Accordingly, we found that the ex vivo differentiation and resorptive activity of OC precursors from TNFi-treated patients were reduced, mainly due to early downregulation of TNF signaling proteins, such as TRAF6 or FRA-2, and to a later reduction of CTSK expression. Moreover, when comparing all studied parameters, we found no differences between the use of monoclonal antibodies (adalimumab, golimumab, and infliximab) and the fusion protein [23], suggesting that they have similar effects on OC precursors. Previous studies have compared the effects of different TNFi in disease activity, sRANKL/OPG ratio, and circulating leukocytes without finding significant differences [24, 25]. It has been previously reported that granulocyte numbers were reduced in circulation after 2 and 14 weeks of infliximab treatment [26]; however, this study identified granulocytes as CD16+ cells instead of CD66b+ cells. We found no significant differences in the frequency of neutrophils and T-lymphocytes or in RANKL surface expression in these cells, but we observed a significant increase in RANKL+ B-lymphocytes accompanied by a decrease in RANKL surface expression. There have been a number of studies addressing the effect of TNFi in RA patients' peripheral lymphocytes; however, there is no consensus among different reports, mainly due to sampling differences. In 2005, Toubi and colleagues have shown that infliximab decreased apoptosis in Tregs of RA patients [27]. Other studies showed that short in vitro exposure of PBMCs to infliximab or etanercept had no effect in peripheral lymphocyte apoptosis [28] or in synovial membrane biopsies [29]. It has previously been shown that RA patients under TNFi have increased number of T-regulatory cells and a reduced number of T-effector cells [30]. Other studies showed that in TNFi-treated RA patients there were no changes in T-regulatory cells frequency [24] or in the frequency of total T-cells, monocytes, or granulocytes and only a transient unspecified effect on B-cells [31]. To our knowledge, a comparative study of RANKL expression in RA patients before and after therapy with TNFi has never been published.
Three monocytes subpopulations, based on their expression of CD14 and CD16 surface markers, have been described in humans [13]. In RA patients, it has been shown that the intermediate subpopulation is increased when compared to healthy donors [13] and apoptosis of local and peripheral monocytes/macrophages was also increased after etanercept or infliximab treatment [29, 32]. Another study has shown no differences in CD14dim or CD14bright subpopulations after 4 months of infliximab therapy [26]. In our cohort, 6 months after TNFi therapy, patients showed decreased classic (CD14brightCD16−) and increased nonclassical (CD14dimCD16+) subpopulations. These changes in frequency were accompanied by a nonsignificant decrease in CD51/CD61 (αvβ3 integrin) and RANK surface expression in all subsets. In accordance with our results, a recent study showed a reduction in classical monocyte subpopulation and an increase in the nonclassical subpopulation following infliximab therapy [33]. Moreover, Sprangers et al. observed that although nonclassical monocytes can also differentiate into OC, these cells have lower resorptive ability [34], which might explain why we did not observe bone resorption increase.
Patients under TNFi had reduced levels of sRANKL, sRANKL/OPG, CTX-I, and P1NP, suggesting a decrease in OC activity and a return to balanced coupling of bone resorption and bone formation. No differences were found in the circulating levels of DKK1 and SOST after TNFi treatment. Previous studies have shown discrepancies in the determination of these bone remodeling-associated proteins. Studies have found no differences in sRANKL or OPG serum levels after infliximab or etanercept [35, 36]. However, contradictory results have emerged regarding both OPG and sRANKL circulating levels after TNFi therapy [37, 38]. DKK1 and sclerostin have a direct effect on bone formation through interaction with the Wnt signaling pathway [39] but they have not been extensively studied in RA patients under TNFi. Previous reports have shown that etanercept has no effect on circulating levels of DKK1 but it increases sclerostin in circulation after treatment [35]. However, infliximab has been shown to decrease DKK1 levels in patients responding to therapy [40]. It has been previously shown that TNFi have a beneficial effect, reducing radiographic damage in RA patients, even in the absence of clinical improvement [9, 41]. Reports have described a decrease in CTX-I or urinary markers of bone resorption after TNFi therapy [35, 42]. However, some discrepancies have been found when studying bone formation markers. Studies with etanercept and infliximab showed no alteration in circulating P1NP levels after treatment [35, 42], while another study with etanercept showed reduced levels of urinary bone formation markers [43].
Although the classical monocyte subpopulation has been considered the OC precursor subset, all three subpopulations can differentiate in vitro into OC [44]. To understand the effect of TNFi in OC differentiation and function, we isolated PBMCs from RA patients before and after TNFi treatment and cultured them in vitro over bone slices. After TNFi treatment, we found a decrease in OC number and both in the total resorbed area and in the average resorbed area per pit. No differences in pit number and in the number of nuclei/OCs, aspects associated with OC activity, were identified [45]. These observations suggest that TNFi reduces the number and mobility of OCs.
Complex in vivo studies with animal models also showed that infliximab and etanercept reduced the bone resorbed area [46, 47] and etanercept decreased αvβ3 integrin expression [48]. In a study similar to ours, Gengenbacher and colleagues studied RA patients under infliximab therapy for 6 months and observed decreased pit number after in vitro cell culture in OC differentiating conditions [36]. There have been reports that infliximab inhibits directly (in vitro) murine and human OC formation [49, 50]. Other authors show that although TNFi reduce the number of murine pre-OCs in vitro, there is no effect in the total number of formed OCs [51]. Another study has shown that infliximab directly inhibits OC formation in high density healthy PBMC cultures without any further stimuli [52]. Etanercept was also shown to inhibit in vitro OC formation induced by M-CSF and IL-23 from healthy subjects [53]. Controversially, Takita and colleagues cultured PBMCs from RA patients, exposing them to M-CSF, RANKL, and infliximab in vitro, and observed that infliximab increased bone resorption when compared to M-CSF and RANKL alone [54].
There is evidence that TNF contributes to expression of specific OC proteins and that it directly activates OC differentiation through cross activation of the NF-κB pathway or c-Jun N-terminal kinase (JNK) signaling cascade [55]. We were interested in understanding the underlying mechanisms of reduced OC formation and bone resorption after TNFi, so we conducted gene expression assays and observed that OC precursors from RA patients after TNFi exposure had decreased expression of TRAF6 at culture day 1, followed by a reduction of FRA-2 and CTSK at day 7, and finally decreased expression of CTSK at culture day 21, when compared to patients before TNFi exposure. RANK/RANKL signaling cross-talks with TNF signaling, as RANK is a TNF-superfamily member [56]. Upon activation, both RANK and TNF activate cytoplasmic kinases and adaptor proteins, including TRAF6, which further activate FRA-2 [57]. FRA-2 is a protein that when associated with Fos and AP-1 promotes the transcription of OC differentiating genes, including CTSK [58]. In TNFi-treated patients, we have observed not only a decrease in serum CTX-I (cleaved by CTSK), but also a reduction in CTSK expression after adhering precursors differentiation in vitro, as well as a decline in the resorbed area/OC. This has previously been observed in a RA patient with concomitant pycnodysostosis, an autosomal recessive mutation in the cathepsin K gene characterized by absence of this enzyme. Osteoclasts from these patients form very small resorbing pits and do not release CTX-I into the culture media [59].
The main limitations of this work were the lack of healthy controls and the reduced number of patients and the diversity of TNF blockers studied, which we tried to overcome by studying the same patient before and after therapy.
Taken together with the results found in the literature, these findings suggest that TNFi decrease bone resorption, independently of the control of disease activity. We propose that this is due to the direct reduction of OC classical precursors and downregulation of intracellular signaling pathways involving TRAF6 resulting in a reduction of CTSK expression and consequent lack of OC motility. Further investigation of the signaling pathways involving TRAF6, such as the ASK1-TRAF6 interaction, is of clear interest in this context.
Supplementary Material
Primers were designed using primer-BLAST software [19] and adhered to the following specifications: they had to be in an exon-exon junction, at annealing temperature of 60°C, with transcript under 150 bp.
Acknowledgments
This work was supported by Fundação para a Ciência e Tecnologia (SFRH/BD/70533/2010 to Inês P. Perpétuo) and by a research grant from Investigator-Initiated Studies Program of Merck Sharp & Dohme Corp. (Merck_P08574 to João E. Fonseca). The authors would like to thank Biobanco-IMM, Lisbon Academic Medical Center, Lisbon, Portugal, for samples collection and storage. The authors would also like to thank Mónica Medina and Soraia Silva for their valuable help in image analysis.
Additional Points
Key Messages. (i) TNFi decrease bone resorption through the direct reduction of OC precursor numbers. (ii) TNFi downregulate the intracellular signaling pathways involving TRAF6 resulting in a reduction of CTSK expression and consequent lack of OC motility.
Disclosure
The opinions expressed in this paper are those of the authors and do not necessarily represent those of Merck Sharp & Dohme Corp. The funding agencies had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing Interests
The authors declare no competing interests regarding the publication of this paper.
References
- 1.Firestein G. S. Evolving concepts of rheumatoid arthritis. Nature. 2003;423(6937):356–361. doi: 10.1038/nature01661. [DOI] [PubMed] [Google Scholar]
- 2.Schett G., Hayer S., Zwerina J., Redlich K., Smolen J. S. Mechanisms of disease: the link between RANKL and arthritic bone disease. Nature Clinical Practice Rheumatology. 2005;1(1):47–54. doi: 10.1038/ncprheum0036. [DOI] [PubMed] [Google Scholar]
- 3.Schett G. Review: immune cells and mediators of inflammatory arthritis. Autoimmunity. 2008;41(3):224–229. doi: 10.1080/08916930701694717. [DOI] [PubMed] [Google Scholar]
- 4.Cascão R., Moura R. A., Perpétuo I., et al. Identification of a cytokine network sustaining neutrophil and Th17 activation in untreated early rheumatoid arthritis. Arthritis Research & Therapy. 2010;12(5, article R196) doi: 10.1186/ar3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Appel H., Loddenkemper C., Miossec P. Rheumatoid arthritis and ankylosing spondylitis—pathology of acute inflammation. Clinical and Experimental Rheumatology. 2009;27(4, supplement 55):S15–S19. [PubMed] [Google Scholar]
- 6.Eng G. P., Bouchelouche P., Bartels E. M., et al. Anti-drug antibodies, drug levels, interleukin-6 and soluble TNF receptors in rheumatoid arthritis patients during the first 6 months of treatment with adalimumab or infliximab: a descriptive Cohort Study. PLoS ONE. 2016;11(9) doi: 10.1371/journal.pone.0162316.e0162316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takeshita M., Suzuki K., Kikuchi J., et al. Infliximab and etanercept have distinct actions but similar effects on cytokine profiles in rheumatoid arthritis. Cytokine. 2015;75(2):222–227. doi: 10.1016/j.cyto.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 8.Zivojinovic S. M., Pejnovic N. N., Sefik-Bukilica M. N., Kovacevic L. V., Soldatovic I. I., Damjanov N. S. Tumor necrosis factor blockade differentially affects innate inflammatory and Th17 cytokines in rheumatoid arthritis. Journal of Rheumatology. 2012;39(1):18–21. doi: 10.3899/jrheum.110697. [DOI] [PubMed] [Google Scholar]
- 9.Hoff M., Kvien T. K., Kälvesten J., Elden A., Kavanaugh A., Haugeberg G. Adalimumab reduces hand bone loss in rheumatoid arthritis independent of clinical response: subanalysis of the PREMIER study. BMC Musculoskeletal Disorders. 2011;12, article 54 doi: 10.1186/1471-2474-12-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aletaha D., Neogi T., Silman A. J., et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League against Rheumatism collaborative initiative. Arthritis and Rheumatism. 2010;62(9):2569–2581. doi: 10.1002/art.27584. [DOI] [PubMed] [Google Scholar]
- 11.Hochberg M. C., Chang R. W., Dwosh I., Lindsey S., Pincus T., Wolfe F. The American College of Rheumatology 1991 revised criteria for the classification of global functional status in rheumatoid arthritis. Arthritis and Rheumatism. 1992;35(5):498–502. doi: 10.1002/art.1780350502. [DOI] [PubMed] [Google Scholar]
- 12.Perpétuo I. P., Raposeiro R., Caetano-Lopes J., et al. Effect of tumor necrosis factor inhibitor therapy on osteoclasts precursors in ankylosing spondylitis. PLoS ONE. 2015;10(12) doi: 10.1371/journal.pone.0144655.e0144655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wong K. L., Yeap W. H., Tai J. J. Y., Ong S. M., Dang T. M., Wong S. C. The three human monocyte subsets: implications for health and disease. Immunologic Research. 2012;53(1–3):41–57. doi: 10.1007/s12026-012-8297-3. [DOI] [PubMed] [Google Scholar]
- 14.Furneri G., Mantovani L. G., Belisari A., et al. Systematic literature review on economic implications and pharmacoeconomic issues of rheumatoid arthritis. Clinical and Experimental Rheumatology. 2012;30(4, supplement 73):S72–S84. [PubMed] [Google Scholar]
- 15.Husheem M., Nyman J. K. E., Vääräniemi J., Vaananen H. K., Hentunen T. A. Characterization of circulating human osteoclast progenitors: development of in vitro resorption assay. Calcified Tissue International. 2005;76(3):222–230. doi: 10.1007/s00223-004-0123-z. [DOI] [PubMed] [Google Scholar]
- 16.Osier L. K., Popoff S. N., Marks S. C., Jr. Osteopetrosis in the toothless rat: failure of osteoclast differentiation and function. Bone and Mineral. 1987;3(1):35–45. [PubMed] [Google Scholar]
- 17.Kurihara N., Suda T., Miura Y., et al. Generation of osteoclasts from isolated hematopoietic progenitor cells. Blood. 1989;74(4):1295–1302. [PubMed] [Google Scholar]
- 18.Arnett T. R., Dempster D. W. A comparative study of disaggregated chick and rat osteoclasts in vitro: effects of calcitonin and prostaglandins. Endocrinology. 1987;120(2):602–608. doi: 10.1210/endo-120-2-602. [DOI] [PubMed] [Google Scholar]
- 19.Ye J., Coulouris G., Zaretskaya I., Cutcutache I., Rozen S., Madden T. L. Primer-BLAST: a tool to design target-specific primers for polymerase chain reaction. BMC Bioinformatics. 2012;13, article 134 doi: 10.1186/1471-2105-13-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wong M. L., Medrano J. F. Real-time PCR for mRNA quantitation. BioTechniques. 2005;39(1):75–85. doi: 10.2144/05391RV01. [DOI] [PubMed] [Google Scholar]
- 21.Caetano-Lopes J., Rodrigues A., Lopes A., et al. Rheumatoid arthritis bone fragility is associated with upregulation of IL17 and DKK1 gene expression. Clinical Reviews in Allergy and Immunology. 2014;47(1):38–45. doi: 10.1007/s12016-013-8366-y. [DOI] [PubMed] [Google Scholar]
- 22.Fransen J., van Riel P. L. C. M. The Disease Activity Score and the EULAR response criteria. Clinical and Experimental Rheumatology. 2005;23(5, supplement 39):S93–S99. [PubMed] [Google Scholar]
- 23.van der Heijde D., Burmester G., Melo-Gomes J., et al. Inhibition of radiographic progression with combination etanercept and methotrexate in patients with moderately active rheumatoid arthritis previously treated with monotherapy. Annals of the Rheumatic Diseases. 2009;68(7):1113–1118. doi: 10.1136/ard.2008.094375. [DOI] [PubMed] [Google Scholar]
- 24.Blache C., Lequerré T., Roucheux A., et al. Number and phenotype of rheumatoid arthritis patients' CD4+CD25hi regulatory T cells are not affected by adalimumab or etanercept. Rheumatology. 2011;50(10):1814–1822. doi: 10.1093/rheumatology/ker183.ker183 [DOI] [PubMed] [Google Scholar]
- 25.Catrina A. I., Af Klint E., Ernestam S., et al. Anti-tumor necrosis factor therapy increases synovial osteoprotegerin expression in rheumatoid arthritis. Arthritis and Rheumatism. 2006;54(1):76–81. doi: 10.1002/art.21528. [DOI] [PubMed] [Google Scholar]
- 26.Coulthard L. R., Geiler J., Mathews R. J., et al. Differential effects of infliximab on absolute circulating blood leucocyte counts of innate immune cells in early and late rheumatoid arthritis patients. Clinical & Experimental Immunology. 2012;170(1):36–46. doi: 10.1111/j.1365-2249.2012.04626.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Toubi E., Kessel A., Mahmudov Z., Hallas K., Rozenbaum M., Rosner I. Increased spontaneous apoptosis of CD4+CD25+ T cells in patients with active rheumatoid arthritis is reduced by infliximab. Annals of the New York Academy of Sciences. 2005;1051:506–514. doi: 10.1196/annals.1361.095. [DOI] [PubMed] [Google Scholar]
- 28.Catrina A. I., Trollmo C., Af Klint E., et al. Evidence that anti-tumor necrosis factor therapy with both etanercept and infliximab induces apoptosis in macrophages, but not lymphocytes, in rheumatoid arthritis joints: extended report. Arthritis and Rheumatism. 2005;52(1):61–72. doi: 10.1002/art.20764. [DOI] [PubMed] [Google Scholar]
- 29.Wijbrandts C. A., Remans P. H., Klarenbeek P. L., et al. Analysis of apoptosis in peripheral blood and synovial tissue very early after initiation of infliximab treatment in rheumatoid arthritis patients. Arthritis and Rheumatism. 2008;58(11):3330–3339. doi: 10.1002/art.23989. [DOI] [PubMed] [Google Scholar]
- 30.Huang Z., Yang B., Shi Y., et al. Anti-TNF-α therapy improves treg and suppresses teff in patients with rheumatoid arthritis. Cellular Immunology. 2012;279(1):25–29. doi: 10.1016/j.cellimm.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 31.Moreland L. W., Bucy R. P., Weinblatt M. E., Mohler K. M., Spencer-Green G. T., Chatham W. W. Immune function in patients with rheumatoid arthritis treated with etanercept. Clinical Immunology. 2002;103(1):13–21. doi: 10.1006/clim.2001.5183. [DOI] [PubMed] [Google Scholar]
- 32.Smeets T. J. M., Kraan M. C., van Loon M. E., Tak P.-P. Tumor necrosis factor α blockade reduces the synovial cell infiltrate early after initiation of treatment, but apparently not by induction of apoptosis in synovial tissue. Arthritis & Rheumatism. 2003;48(8):2155–2162. doi: 10.1002/art.11098. [DOI] [PubMed] [Google Scholar]
- 33.Aeberli D., Kamgang R., Balani D., Hofstetter W., Villiger P. M., Seitz M. Regulation of peripheral classical and non-classical monocytes on infliximab treatment in patients with rheumatoid arthritis and ankylosing spondylitis. RMD Open. 2016;2(1) doi: 10.1136/rmdopen-2015-000079.e000079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sprangers S., Schoenmaker T., Cao Y., Everts V., de Vries T. J. Different blood-borne human osteoclast precursors respond in distinct ways to IL-17A. Journal of Cellular Physiology. 2016;231(6):1249–1260. doi: 10.1002/jcp.25220. [DOI] [PubMed] [Google Scholar]
- 35.Lim M. J., Kwon S. R., Joo K., Son M. J., Park S.-G., Park W. Early effects of tumor necrosis factor inhibition on bone homeostasis after soluble tumor necrosis factor receptor use. Korean Journal of Internal Medicine. 2014;29(6):807–813. doi: 10.3904/kjim.2014.29.6.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gengenbacher M., Sebald H.-J., Villiger P. M., Hofstetter W., Seitz M. Infliximab inhibits bone resorption by circulating osteoclast precursor cells in patients with rheumatoid arthritis and ankylosing spondylitis. Annals of the Rheumatic Diseases. 2008;67(5):620–624. doi: 10.1136/ard.2007.076711. [DOI] [PubMed] [Google Scholar]
- 37.González-Alvaro I., Ortiz A. M., Tomero E. G., et al. Baseline serum RANKL levels may serve to predict remission in rheumatoid arthritis patients treated with TNF antagonists. Annals of the Rheumatic Diseases. 2007;66(12):1675–1678. doi: 10.1136/ard.2007.071910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ziolkowska M., Kurowska M., Radzikowska A., et al. High levels of osteoprotegerin and soluble receptor activator of nuclear factor κB ligand in serum of rheumatoid arthritis patients and their normalization after anti–tumor necrosis factor α treatment. Arthritis and Rheumatism. 2002;46(7):1744–1753. doi: 10.1002/art.10388. [DOI] [PubMed] [Google Scholar]
- 39.Miao C.-G., Yang Y.-Y., He X., et al. Wnt signaling pathway in rheumatoid arthritis, with special emphasis on the different roles in synovial inflammation and bone remodeling. Cellular Signalling. 2013;25(10):2069–2078. doi: 10.1016/j.cellsig.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 40.Wang S.-Y., Liu Y.-Y., Ye H., et al. Circulating dickkopf-1 is correlated with bone erosion and inflammation in rheumatoid arthritis. Journal of Rheumatology. 2011;38(5):821–827. doi: 10.3899/jrheum.100089. [DOI] [PubMed] [Google Scholar]
- 41.Seriolo B., Paolino S., Sulli A., Cutolo M. Are there any positive effects of TNF-α blockers on bone metabolism? Reumatismo. 2006;58(3):199–205. doi: 10.4081/reumatismo.2006.199. [DOI] [PubMed] [Google Scholar]
- 42.Chopin F., Garnero P., Le Henanff A., et al. Long-term effects of infliximab on bone and cartilage turnover markers in patients with rheumatoid arthritis. Annals of the Rheumatic Diseases. 2008;67(3):353–357. doi: 10.1136/ard.2007.076604. [DOI] [PubMed] [Google Scholar]
- 43.Yasunori K., Masaaki T., Tetsuyuki N., Hayato K., Akira N. Reduction of urinary levels of pyridinoline and deoxypyridinoline and serum levels of soluble receptor activator of NF-kappaB ligand by etanercept in patients with rheumatoid arthritis. Clinical Rheumatology. 2008;27(9):1093–1101. doi: 10.1007/s10067-008-0870-8. [DOI] [PubMed] [Google Scholar]
- 44.Costa-Rodrigues J., Fernandes A., Fernandes M. H. Spontaneous and induced osteoclastogenic behaviour of human peripheral blood mononuclear cells and their CD14+ and CD14− cell fractions. Cell Proliferation. 2011;44(5):410–419. doi: 10.1111/j.1365-2184.2011.00768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Piper K., Boyde A., Jones S. J. The relationship between the number of nuclei of an osteoclast and its resorptive capability in vitro. Anatomy and Embryology. 1992;186(4):291–299. doi: 10.1007/BF00185977. [DOI] [PubMed] [Google Scholar]
- 46.Matsuno H., Yoshida K., Ochiai A., Okamoto M. Requirement of methotrexate in combination with anti-tumor necrosis factor-alpha therapy for adequate suppression of osteoclastogenesis in rheumatoid arthritis. Journal of Rheumatology. 2007;34(12):2326–2333. [PubMed] [Google Scholar]
- 47.Childs L. M., Goater J. J., O'Keefe R. J., Schwarz E. M. Efficacy of etanercept for wear debris-induced osteolysis. Journal of Bone and Mineral Research. 2001;16(2):338–347. doi: 10.1359/jbmr.2001.16.2.338. [DOI] [PubMed] [Google Scholar]
- 48.Terry S. Y. A., Koenders M. I., Franssen G. M., et al. Monitoring therapy response of experimental arthritis with radiolabeled tracers targeting fibroblasts, macrophages, or integrin αvβ3. Journal of Nuclear Medicine. 2016;57(3):467–472. doi: 10.2967/jnumed.115.162628. [DOI] [PubMed] [Google Scholar]
- 49.Yago T., Nanke Y., Ichikawa N., et al. IL-17 induces osteoclastogenesis from human monocytes alone in the absence of osteoblasts, which is potently inhibited by anti-TNF-α antibody: a novel mechanism of osteoclastogenesis by IL-17. Journal of Cellular Biochemistry. 2009;108(4):947–955. doi: 10.1002/jcb.22326. [DOI] [PubMed] [Google Scholar]
- 50.Lee C.-K., Lee E. Y., Chung S. M., Mun S. H., Yoo B., Moon H.-B. Effects of disease-modifying antirheumatic drugs and antiinflammatory cytokines on human osteoclastogenesis through interaction with receptor activator of nuclear factor κB, osteoprotegerin, and receptor activator of nuclear factor κB ligand. Arthritis & Rheumatism. 2004;50(12):3831–3843. doi: 10.1002/art.20637. [DOI] [PubMed] [Google Scholar]
- 51.Binder N. B., Puchner A., Niederreiter B., et al. Tumor necrosis factor-inhibiting therapy preferentially targets bone destruction but not synovial inflammation in a tumor necrosis factor-driven model of rheumatoid arthritis. Arthritis and Rheumatism. 2013;65(3):608–617. doi: 10.1002/art.37797. [DOI] [PubMed] [Google Scholar]
- 52.de Vries T. J., Yousovich J., Schoenmaker T., Scheres N., Everts V. Tumor necrosis factor-α antagonist infliximab inhibits osteoclast formation of peripheral blood mononuclear cells but does not affect periodontal ligament fibroblast-mediated osteoclast formation. Journal of Periodontal Research. 2016;51(2):186–195. doi: 10.1111/jre.12297. [DOI] [PubMed] [Google Scholar]
- 53.Yago T., Nanke Y., Kawamoto M., et al. IL-23 induces human osteoclastogenesis via IL-17 in vitro, and anti-IL-23 antibody attenuates collagen-induced arthritis in rats. Arthritis Research and Therapy. 2007;9(5, article R96) doi: 10.1186/ar2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Takita C., Fujikawa Y., Itonaga I., Taira H., Kawashima M., Torisu T. Infliximab acts directly on human osteoclast precursors and enhances osteoclast formation induced by receptor activator of nuclear factor κB ligand in vitro. Modern Rheumatology. 2005;15(2):97–103. doi: 10.1007/s10165-004-0373-7. [DOI] [PubMed] [Google Scholar]
- 55.Kobayashi K., Takahashi N., Jimi E., et al. Tumor necrosis factor α stimulates osteoclast differentiation by a mechanism independent of the ODF/RANKL-RANK interaction. Journal of Experimental Medicine. 2000;191(2):275–286. doi: 10.1084/jem.191.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Osta B., Benedetti G., Miossec P. Classical and paradoxical effects of TNF-α on bone homeostasis. Frontiers in Immunology. 2014;5, article 48 doi: 10.3389/fimmu.2014.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Teitelbaum S. L., Ross F. P. Genetic regulation of osteoclast development and function. Nature Reviews Genetics. 2003;4(8):638–649. doi: 10.1038/nrg1122. [DOI] [PubMed] [Google Scholar]
- 58.Bozec A., Bakiri L., Hoebertz A., et al. Osteoclast size is controlled by Fra-2 through LIF/LIF-receptor signalling and hypoxia. Nature. 2008;454(7201):221–225. doi: 10.1038/nature07019. [DOI] [PubMed] [Google Scholar]
- 59.Ainola M., Valleala H., Nykänen P., Risteli J., Hanemaaijer R., Konttinen Y. T. Erosive arthritis in a patient with pycnodysostosis: an experiment of nature. Arthritis and Rheumatism. 2008;58(11):3394–3401. doi: 10.1002/art.23996. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Primers were designed using primer-BLAST software [19] and adhered to the following specifications: they had to be in an exon-exon junction, at annealing temperature of 60°C, with transcript under 150 bp.