Figure 2.
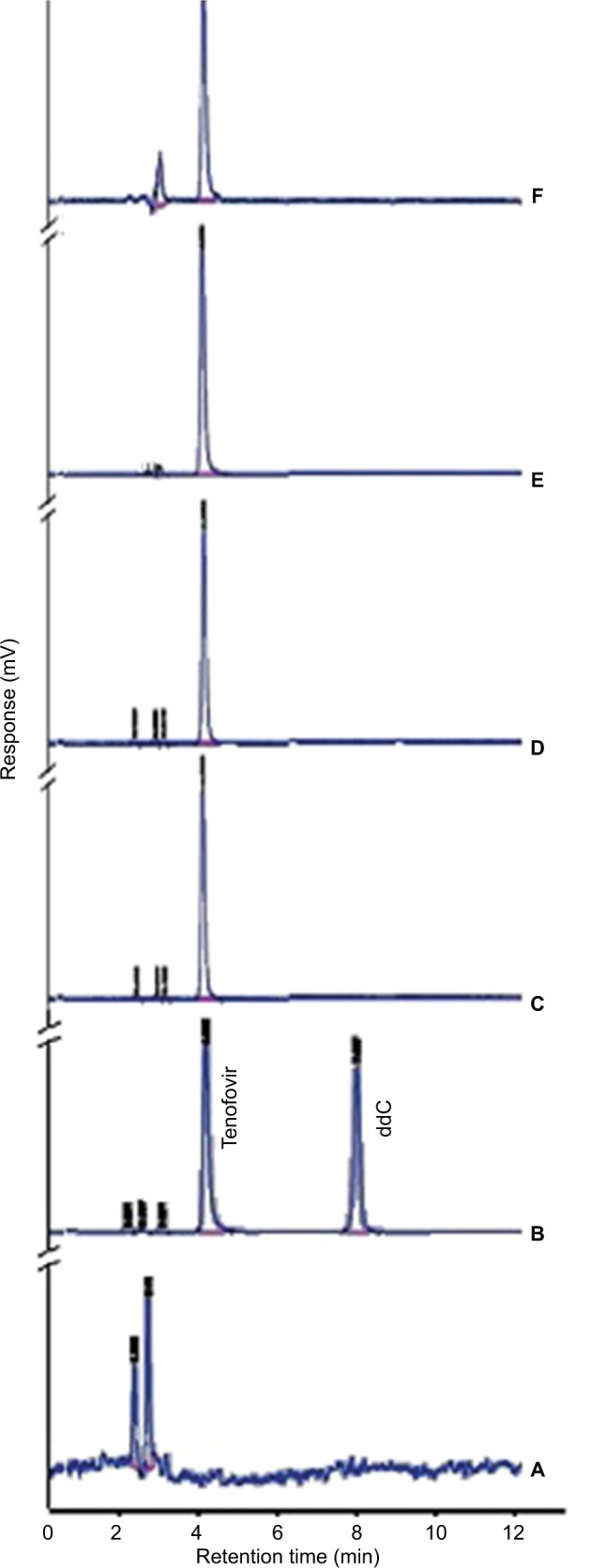
HPLC chromatograms for (A) blank, (B) system suitability (6 µg/mL tenofovir and 6 µg/mL ddC), (C) EF sample of formulation A, (D) EF sample of formulation B, (E) Caco-2 study sample of formulation A, and (F) Caco-2 study sample of formulation B. Formulations were prepared using 50 mg cholesterol, either 7.5% (formulation A) or 15% (formulation B) stearylamine as a positive charge imparting agent and an amount of phospholipon 100H to make a total lipid pool of 150 mg.
Abbreviations: HPLC, high-performance liquid chromatography; EF, entrapment efficiency; ddC, dideoxy-cytosine.
