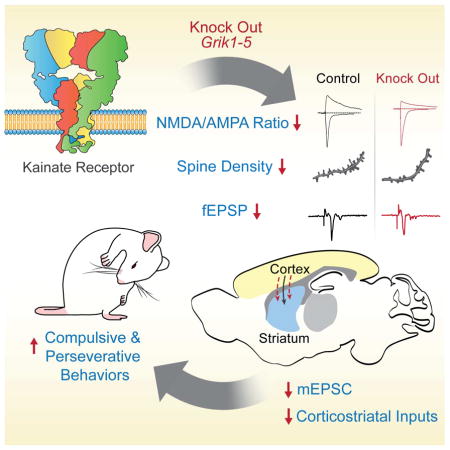
Summary
Kainate receptors are members of the glutamate receptor family, that regulate synaptic function in the brain. They modulate synaptic transmission and the excitability of neurons; however, their contributions to neural circuits that underlie behavior are unclear. To understand the net impact of kainate receptor signaling, we generated knockout mice in which all five kainate receptor subunits were ablated (5ko). These mice displayed compulsive and perseverative behaviors including over grooming, as well as motor problems, indicative of alterations in striatal circuits. There were deficits in corticostriatal input to spiny projection neurons (SPNs) in the dorsal striatum and correlated reductions in spine density. The behavioral alterations were not present in mice only lacking the primary receptor subunit expressed in adult striatum (GluK2 ko), suggesting that signaling through multiple receptor types is required for proper striatal function. This demonstrates that alterations in striatal function dominate the behavioral phenotype in mice without kainate receptors.
Keywords: kainate receptor, striatum, perseverative behavior, corticostriatal synapse
Graphical Abstract
Introduction
Kainate receptors (KARs) are glutamate-gated receptors that are expressed ubiquitously throughout the brain but with distinct expression patterns for the individual subunits (Wisden and Seeburg, 1993). The endogenous receptors are heteromultimeric proteins comprised of combinations of the GluK1-GluK5 subunits that form ligand-gated ion channel receptors and link to non-ionotropic signaling pathways (Contractor et al., 2011). Kainate receptors play a multitude of roles at synapses, primarily as modulators of synaptic transmission, although in some neurons they also mediate postsynaptic currents. The diversity in their subunit composition and localization combined with multiple signaling modalities has complicated analysis of their roles in the brain. This has led to a lack of clarity in understanding their principal contributions to both circuit and behavioral functions. Likewise, genetic linkage has been demonstrated to several neuropsychiatric and neurodevelopmental disorders, including schizophrenia (Begni et al., 2002; Pickard et al., 2006), obsessive compulsive disorder (OCD)(Delorme et al., 2004; Sampaio et al., 2010), autism (Jamain et al., 2002) and mental retardation (Motazacker et al., 2007), but in most cases these have yet to receive strong confirmatory support (Lerma and Marques, 2013).
Analysis of single subunit kainate receptor knockout mice has been helpful in deciphering some of these roles both at the synaptic (Contractor et al., 2003; Mulle et al., 1998; Mulle et al., 2000) and behavioral level (Catches et al., 2011; Shaltiel et al., 2008). Expression of the subunits varies at different developmental time points in many cells (Bahn et al., 1994), and therefore the interpretation of behavioral studies is complicated by difficulties in verifying if complete functional loss of kainate receptors occurs in any specific cell type throughout development in these monogenic mutants. This caveat is even more relevant given that there is not an obligate subunit required for the expression of the kainate receptor heteromultimeric complex. Therefore, multiple genes must be targeted in mice to ensure functional elimination of kainate receptors. To achieve this goal, we intercrossed five mice with targeted mutations in the kainate receptor genes, which are separately located on chromosomes 16, 10, 4, 9, and 7, to produce a mouse with all five kainate receptor genes Grik1-5 disrupted (5ko mice). These mice are the first viable strain with a complete family class of ionotropic glutamate receptor ablated. Generation of these mice has allowed us to study both behavioral and synaptic alterations when kainate receptors are genetically disrupted throughout the life of the animal.
We found that 5ko mice had striking behavioral alterations that were predominantly indicative of disrupted corticostriatal function. The behavioral alterations in 5ko mice were exemplified by self-injurious over-grooming, an increase in perseverative behaviors, and impairments in motor ability. Moreover, we found that corticostriatal synaptic transmission was impaired in 5ko mice; spine density was decreased in both the D1 (direct pathway) and D2 (indirect pathway) spiny projection neurons (SPNs) with a concomitant decrease in the frequency of mEPSC events. Together, study of these mice has uncovered a previously unknown role for kainate receptors in the proper functioning of striatal synapses and suggests that kainate receptors have a major role in the correct formation of corticostriatal circuits.
Results
Ablation of all kainate receptor subunits in 5ko mice
To determine how loss of kainate receptor complexes will affect neurological phenotypes in a mouse, we intercrossed each of the single gene targeted mice (Contractor et al., 2003; Fernandes et al., 2009; Mulle et al., 1998; Mulle et al., 2000; Pinheiro and Mulle, 2008). Mice lacking all five KAR genes (5ko) mice were viable and homozygous mice were maintained on a C57BL/6:129S background. Heterozygous pups (5het) from a wildtype (WT) female in the same cage were used as a control. Successful elimination of all five genes was confirmed by genotyping and western blot (Figure S1A). Mortality during the perinatal period was unaltered, despite a consistent decrease in body weight in 5ko mice (Student’s t-test, p < 0.01, n =20 5het, n = 37 5ko) (Figure S1B). Likewise MRI analysis did not demonstrate wide-scale alteration in absolute volumes in regions including the striatum, hippocampus and motor cortex (Figure S1 C–F).
5ko mice develop facial lesions through self-injurious over-grooming
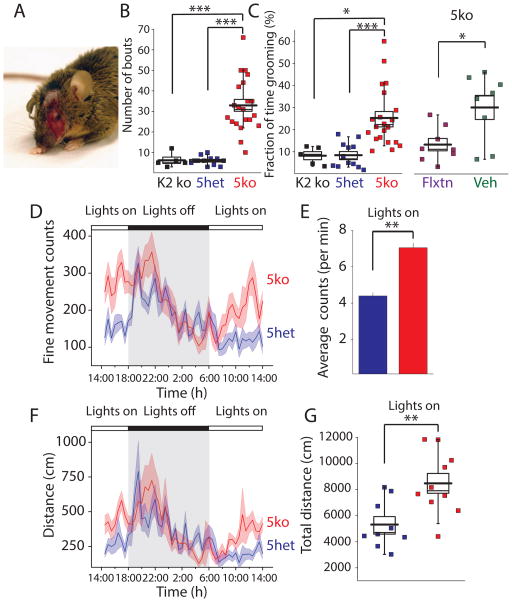
While loss of kainate receptors throughout the CNS could potentially result in deficits in a number of behaviors, we found the most prominent effect on behavior in 5ko mice was evident starting at 4 to 6 months when a subset of mice developed facial lesions due to self-injurious over grooming behavior (Figure 1A). This was quantified as the number of bouts and the time spent grooming by singly housed male mice (4 months old) monitored in a novel cage for a period of 30 minutes. For 5ko mice both measures were significantly enhanced in comparison to 5het mice (p < 0.001, Student’s t-test, n = 21 5ko, n = 12 5het) (Figure 1B & C). We found no evidence of altered grooming behavior in mice lacking GluK2 (n = 7), the primary receptor subunit expressed in the striatum of adult mice, a region critical to grooming behavior (Ahmari et al., 2013; Welch et al., 2007). Prior work has demonstrated that excessive grooming in genetic or induced mouse models of OCD can be reduced by the administration of selective serotonin reuptake inhibitors (SSRIs) (Ahmari et al., 2013; Welch et al., 2007), a first line monotherapy for OCD. Eight week chronic administration of fluoxetine (Flxtn) to 5ko mice (6mg/100ml in drinking water ad libitum) significantly reduced grooming in 5ko mice compared to a vehicle cohort treated over the same time period (p < 0.05, Student’s t-test, n = 8 per group) (Figure 2C). To quantify repetitive behaviors without the potential complication of elevated anxiety in a novel environment, we monitored mice in automated phenotyping cages that allow for extended monitoring of undisturbed activity over days and across the circadian cycle in a habituated environment. Male mice (4–6 months, 5ko n = 10, 5het n = 9) were housed in cages for 6 days and after 2 days of habituation, data were collected for 4 days. We quantified total ambulation or locomotion, as well as the fine movement counts that detect all stationary non-ambulatory repetitive movement including grooming. Consistent with manual quantification, automated counting detected more non-ambulatory activity, fine movement counts, in 5ko mice (F1,17 = 8.63, Two-Way RM ANOVA, p < 0.01) (Figure 1D). The increase in repetitive non-ambulatory activity varied across the circadian cycle with similar counts for both groups during the dark cycle but consistently elevated counts for the 5ko group during the light period. Total activity during the lights-on period alone was significantly higher in the 5ko group compared to controls (Student t-test, p < 0.01) (Figure 1E). Elevated fine movement activity was paralleled by an increase in total ambulatory movement of the 5ko mice compared to 5het controls over the course of the four testing days (Figure 1F). Again, the biggest differences appeared during the lights-on period (Figure 1F & G) (Student t-test, p < 0.01). These data further revealed a difference in the circadian pattern of activity and demonstrated that 5ko mice have increased fine movement, including grooming, and ambulatory locomotion activity, particularly during the light cycle when their control 5het counterparts have low activity levels. As in human compulsive disorders, elevated compulsive behavior in mice can be accompanied by increased anxiety-related behavior (Shmelkov et al., 2010; Welch et al., 2007). However, we found no evidence of increased anxiety-like behavior in the 5ko mice in both the open field test (F1,23 = 0.62, Two-Way RM ANOVA, p > 0.05 Figure S2A; Student t-test. p > 0.05 Figure S2B) and the elevated zero maze (F1,15 = 1.295, Two-Way RM ANOVA, p > 0.05, Figure S2D; Student t-test p > 0.05 Figure S2E).
Figure 1. 5ko Mice Demonstrate Elevated Self-Grooming Behavior.
(A) Representative picture of a singly housed male mouse that developed grooming induced facial lesions (B) Manually quantified grooming bouts in GluK2 single ko mice, 5het and 5ko mice. (C) Left panel: Fraction of time spent grooming. Mice were monitored by video camera for 30 minutes and 10 minutes of the recording was manually scored with the observer blind to genotype. Right panel: Effect of chronic fluoxetine (Flxtn) administration to 5ko mice (D & E) Fine movement counts quantified during the circadian cycle by automated monitoring of mice. The activity over the light cycle is represented for the 4 day period. Elevated fine movement counts were observed during the lights on period in 5ko cohort. (F & G) Ambulatory locomotion measured as the distance moved (30 min bins) during the circadian cycle monitored over 4 days.
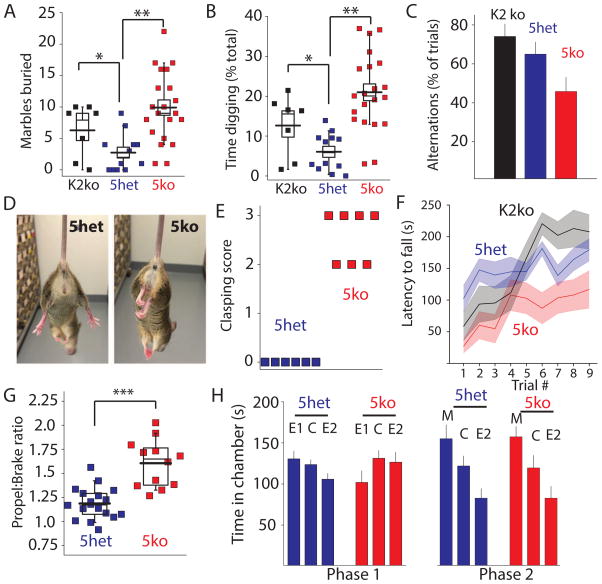
Figure 2. Striatal Dependent Perseverative and Motor Behaviors are Aberrant in 5ko mice.
(A & B) Marbles buried and digging time quantified over 15 minutes in 5ko mice, 5het, and GluK2 ko mice. (C) Grouped data of spontaneous alternations in the Y maze. (D) Representative pictures of hindlimb clasping in 5het and 5ko mice and (E) quantification of hindlimb clasping score (F) Latency to fall measured on the accelerating rotarod test. (G) Gait analysis of mice demonstrating an increased propel:brake ratio in 5ko mice (H) Quantification of 3 chamber social interaction task. In Phase 1 when both wings of the chamber are empty (E1 and E2) mice spend equivalent time in each chamber and center (C). In phase 2 a stranger mouse is placed in one chamber (M).
5ko mice have alterations in perseveration and other striatal dependent behaviors
Over-grooming in mice can be a manifestation of elevated perseverative behaviors; we therefore performed additional tests to measure perseveration in 5ko mice. In the marble burying task, which is a measure of digging perseveration and repetitive behavior (Thomas et al., 2009), we quantified total digging time and the subsequent number of marbles buried in the bedding. The 5ko cohort buried significantly more marbles and spent a greater fraction of time during the test digging in comparison to the 5het control cohort (p < 0.001 ANOVA, with Dunn-Sidak, n = 21 5ko, n=12 5het) (Figure 2A & B). Surprisingly, single GluK2 ko mice (n = 7) also had elevated marble burying and increased digging time in comparison to the 5het group (Figure 2A & B). As an additional test of perseverative behavior, we measured spontaneous alternations made by mice in the Y-maze (Yadin et al., 1991). In successive trials both GluK2 ko and 5het mice chose an alternate arm in the majority of second trials, reflecting a natural exploratory behavior in rodents (Figure 2C). However, 5ko mice showed no preference for the alternative arm on a successive trial, selecting it in 45.8 ± 7.3%, of trials (Figure 2C) (p < 0.05, Kruskall-Wallis with Mann-Whitney U-test, n = 8 per group). Therefore, consistent with an elevated perseverative behavior phenotype, 5ko mice showed a significant decrease in preference for the alternate arm compared to GluK2 ko and 5het control mice.
The striatum has extensive connectivity with many different cortical regions, from prefrontal and association cortexes to sensorimotor regions. Consequently, alterations in striatal activity can lead to both increased perseverative behaviors (Welch et al., 2007) and changes in simple motor function, leading us to perform measures of motor coordination in the 5ko mice. We found that a classic hind limb clasping response to tail suspension, which has been observed in mice with striatal lesions (Zhang et al., 2014), was prominent in 5ko mice. Quantifying this behavior using a phenotype scoring system (Guyenet et al., 2010), demonstrated that all 5ko mice showed hind limb clasping and scored 2 or 3 on the clasping scale. None of the 5het littermates showed any tail suspension-induced clasping (Figure 2D & E) similar to WT animals and GluK2 ko mice (not shown). In addition, in a motor learning task on the accelerating rotarod the performance of 5ko mice was significantly impaired in comparison to 5het and GluK2 ko groups (Two-Way ANOVA, F1,16 = 9.73, P < 0.001 for 5ko vs 5het; Two-Way ANOVA, F1,18 = 9.25, P < 0.01 for 5ko vs GluK2 ko)(Figure 2F); there was no difference in performance between GluK2 ko and 5het mice (Two-Way ANOVA, F1, 8 = 0.94, p > 0.05).
For a detailed kinematic gait analysis mice were placed on a treadmill and parameters of stride extracted from the digitized fore and hind paw patterns at a fixed walking velocity of 14 cm s−1. There was no difference between 5het (n = 18) and 5ko (n = 12) mice in most stride parameters (See Table 1). However, 5ko mice displayed a significant decrease in brake time, (time from initial paw contact finishing the swing phase to maximum paw contact) (Student’s t-test, p < 0.05), and an increase in propel time) (time from maximum paw contact to the start of the swing phase) (Student’s t-test, p < 0.0001). Consequently, 5ko mice had a significantly higher propel:brake ratio than 5het controls (Figure 2G). Similar changes have been observed in mouse models of Huntington’s disease, and demonstrate that 5ko mice have altered rhythmicity in their gate (Wright et al., 2015).
The behavioral phenotype in 5ko mice strongly suggests that there are alterations in striatal function. Corticostriatal circuitry disruption is often also associated with the core features of autism (Peca et al., 2011), including the perseverative behavior that we have observed in 5ko mice. Therefore, we tested whether 5ko mice displayed abnormalities in social interaction, another autism-linked behavior. In a three-chamber social interaction assay during the habituation (Phase 1) when both chambers contained empty wired cups (E), both control groups (n = 12) and 5ko mice (n = 9) spent a similar amount of time in each of the compartment (Figure 2H, paired Student’s t-test, p > 0.05). When a novel social partner was introduced into one side (Phase 2), both groups spent more time in the mouse-containing chamber (M) than the empty cup (E) (paired Student’s t-test, p < 0.01), and there was no significant difference between the control group and 5ko group (Figure 2H, Phase 2) (Student’s t-test, p > 0.05). Together these experiments demonstrate that 5ko mice have multiple alterations in perseverative behavior and motor coordination, but there is dissociation from social interaction behavior that is often disrupted by altering corticostriatal activity (Mei et al., 2016).
Corticostriatal Synaptic Transmission is altered in 5ko mice
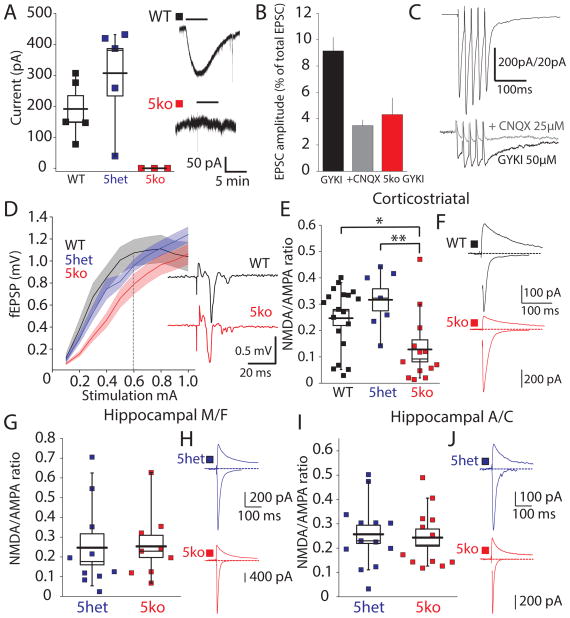
The overt behavioral alterations in the 5ko mice are suggestive of aberrant striatal function that is emergent when all the kainate receptor subunits are eliminated. In juvenile mice the GluK2 subunit is the principal subunit expressed in both D1 and D2 expressing spiny projection neurons (SPNs), which are the major class of projection neuron in the striatum (Chergui et al., 2000). To confirm the presence of kainate receptors in SPNs, we made single cell whole-cell voltage clamp recordings from visually identified neurons in acute slices of the dorsal striatum. Application of kainic acid (10 μM) resulted in inward currents in SPN recordings from WT and 5het mice, whereas no current was detected in recordings from 5ko animals (Figure 3A). Unlike AMPA receptors, localization of kainate receptors to postsynaptic densities is not ubiquitous in all neurons throughout the CNS. Prior studies have found that there is a slow synaptic current in SPNs that was not inhibited by AMPA and NMDA receptor antagonism (Chergui et al., 2000) but which was reduced by the relatively selective kainate receptor blocker ACET (Vizcarra-Chacon et al., 2013). We confirmed that kainate receptors contribute to corticostriatal synaptic transmission by mediating a small amplitude GYKI53655-resistant current that was partially blocked by the non-selective AMPA/kainate receptor antagonist CNQX (n = 20 WT, n = 3 5ko) (Figure 3B & C). The GYKI53655-resistant current in 5ko recordings was of equivalent amplitude to the remaining current in WT mice after CNQX (Figure 3B), further demonstrating that kainate receptors contribute to synaptic transmission at these synapses.
Figure 3. Corticostriatal Synaptic Function is Altered in 5ko mice.
(A) Agonist induced kainate receptor mediated currents in SPNs elicited by bath applied kainic acid (10μM). (B) Kainate mediated synaptic current activated by stimulation of corticostriatal inputs to SPNs. Residual current in GYKI53655 (AMPA receptor antagonist) represents kainate component of the compound EPSC. Addition of CNQX (AMPA/kainate receptor antagonist) further inhibits this component in WT mice (grey), whereas no kainate receptor mediated current observed in 5ko mice (red) (C) Representative traces from a WT recording (D) Input-output relationship of striatal field response. (E) NMDA:AMPA ratio recorded in voltage clamp experiments from SPNs in WT, 5het and 5ko mice. A significant reduction was observed in 5ko mice (F) Representative traces from recordings from WT and 5ko mice. (G) NMDA:AMPA ratio of mossy fiber (MF) synapses recorded in CA3 neurons. (H) Representative MF EPSC traces recorded at +40mV and −70mV Vm. (I) NMDA:AMPA ratio of association/commissural (A/C) synapses in CA3 neurons. (J) Representative A/C EPSCs
We tested whether corticostriatal function is disrupted in 5ko mice in field recordings of inputs to the dorsal striatum. Input-output relationships for corticostriatal transmission in 5ko and control mice demonstrated that field population response amplitudes were significantly reduced in the 5ko mice in comparison to both WT mice (Two-way ANOVA, F1,30 = 7.96, p < 0.01) and 5het controls (F1,27 = 5.59, p < 0.05), whereas there was no significant difference in the field response between WT and 5het mice (F1,23 = 0.57, p > 0.05) (Figure 3D). As an additional measure of synaptic integrity, we made voltage clamp recordings from visually identified SPNs and isolated corticostriatal excitatory postsynaptic currents (EPSCs). NMDA receptor and AMPA receptor EPSCs were measured by recording at +40mV and −70mV respectively. The NMDA:AMPA amplitude ratio for WT mice (0.247 ± 0.028, n = 19) and for 5het mice (0.317 ± 0.042, n = 7) were significantly higher in 5ko mice (0.128 ± 0.036, n = 13, p < 0.05 5ko comparison, p < 0.01 5het comparison Student’s t-test)(Figure 3E & F), further supporting alterations in corticostriatal synapses in 5ko mice. To determine whether this aberrant synaptic function is present in other brain regions, we recorded from synapses on CA3 neurons of the hippocampus. Kainate receptors are present in the postsynaptic densities at the mossy fiber (MF) input on the proximal dendrites of CA3 neurons, but excluded from the more distal associational/commissural (A/C) synapses (Straub et al., 2016). NMDA:AMPA ratios recorded from 5ko mice were not significantly different to those in 5het mice in either of these synaptic inputs (n = 13 A/C 5het, n = 12 A/C 5ko; n = 10 5het MF, n = 9 5ko MF) (Figure 3G–J), suggesting that kainate receptors play differential roles in the development and stabilization of synaptic contacts in different neuronal types.
Striatal synapse density is reduce in 5ko mice
Kainate receptors interact with several components of the postsynaptic density (PSD) including PSD95 (Garcia et al., 1998). Because the 5ko mice show behavioral phenotypes similar to those in Sapap3 ko mice (Welch et al., 2007), we tested for an association between these proteins. In a recombinant expression system, the GluK2 subunit interacted with Sapap3 only when PSD95 was cotransfected (Figure S3A). Endogenous Sapap was also detected after co-immunoprecipitation with both GluK2 and PSD95 from striatal homogenates (Figure S3B). Because these data demonstrate that kainate receptors are an integral part of the postsynaptic complex in striatal synapses, it is possible that loss of the receptors can lead to disorganization of the PSD. Electron micrographs of tissue from the dorsal striatum did not reveal any difference in the PSD length of individual synapses when comparing 5het and 5ko mice (Figure S3 C & D); however, the PSD thickness was significantly reduced in 5ko mice (5het n = 59, 5ko n = 74, p < 0.01 Kolmogorov-Smirnov, KS test) (Figure S3 E).
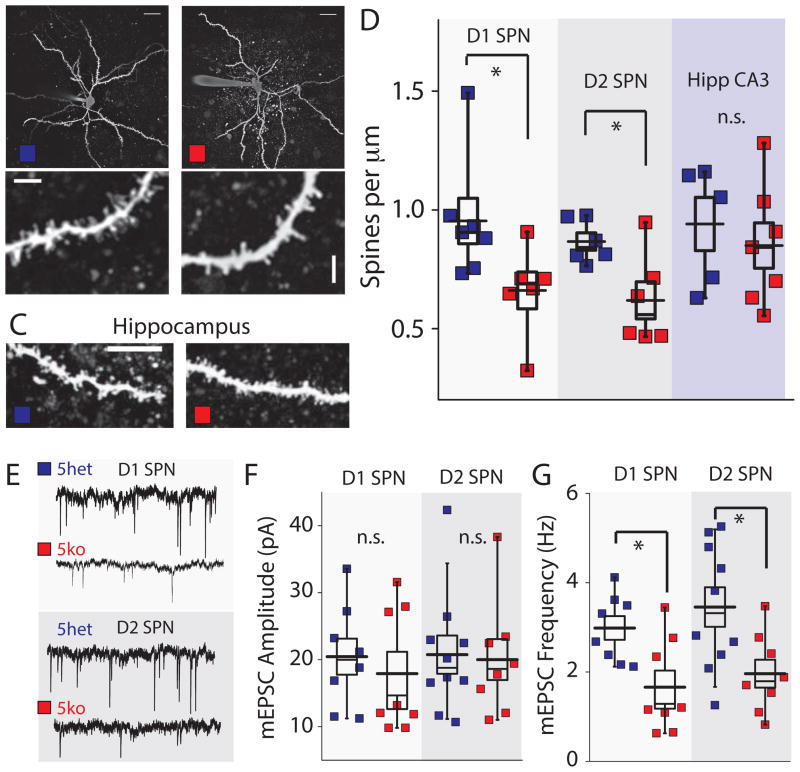
To further investigate alterations in SPN synapses we performed two-photon imaging of live SPNs in dorsal striatal slices, and used post hoc molecular characterization to identify the neuron type unequivocally. SPNs can be segregated into two populations based upon their projections to the substantia nigra pars reticulata (D1R expressing) or globus pallidus (D2R expressing) in the basal ganglia (Gerfen and Surmeier, 2011). We collected the cytoplasmic contents of each recorded neuron and performed single cell RT-PCR for markers of D1 AND D2 SPNs (See methods). Analysis of spine density (secondary and tertiary dendritic segments 50–200 μm from the soma) revealed a significant decrease in spine number in 5ko mice in both D1 (n = 7 5het, n = 6 5ko) and D2 identified cell types (n = 6 per group) (Student’s t-test p < 0.05) (Figure 4A–B & D). In contrast, analysis of dendritic segments of CA3 pyramidal neurons in the hippocampus showed no difference in the density of CA3 spines in the 5ko mice (n = 7 5ko, n = 5 5het), suggesting a specific effect of loss of kainate receptors on striatal synapses (Figure 4C & D). A reduction in spine density may result in a decrease in the number of excitatory synapses; therefore, we performed electrophysiological recordings from post hoc identified SPNs to measure miniature EPSCs (mEPSCs). We found that there was a significant decrease in the frequency of mEPSC events in both D1 and D2 SPNs in 5ko mice (p < 0.05 Student’s t-test, n = 10 D2 5het, n = 8 D2 5ko, n = 8 D1 5het, n = 8 D1 5ko)(Figure 4E & G), whereas the amplitude of recorded events was not different in either cell type (Figure 4E & F). These results demonstrate that there are both functional and structural alterations in synapses in the striatum and, together with the behavioral data, show that loss of all kainate receptor subunits has a major impact on corticostriatal function.
Figure 4. Spine Density and mEPSC Frequency are Reduced in Both Direct and Indirect Pathway SPNs in 5ko Mice.
(A & B) Representative two-photon laser scanning microscopy (2PLSM) images of D1 SPN in 5het or 5ko mouse slices filled with AlexaFluor 568 through the patch pipette. Lower panels show higher magnification of dendritic regions outlined by boxes in the maximum projection images of the whole neuron (C) Representative images of dendritic sections from hippocampal CA3 neurons from 5het (left) and 5ko (right) (D) Analysis of spine density in D1 and D2 SPNs and CA3 pyramidal neurons in 5het (blue) and 5ko (red) mice. (E) Example traces of mEPSCs recorded in SPNs in 5het and 5ko slices. (F) Analysis of mEPSC amplitude in each recorded and positively identified cell type. No genotype-associated difference is observed in either D1 or D2 SPNs (G) Analysis of mEPSC frequency in D1 and D2 SPNs demonstrating a significant reduction in frequency of events in 5ko neurons.
Discussion
Emergent perseverative behavior and motor dysfunction in 5ko mice
A growing number of mutations in glutamate receptor genes have been associated with neurological and neuropsychiatric conditions, which is consistent with their prominent role in mediating and modulating synaptic transmission. Genetic analysis studies indicate that kainate receptors might be involved in the pathogenesis of neuropsychiatric diseases linked to striatal dysfunction, including OCD (Delorme et al., 2004; Mattheisen et al., 2015; Sampaio et al., 2010). Analysis of individual kainate receptor subunit mutant mice provided experimental support for some of these genetic associations and discovered significant behavioral alterations consistent with roles for altered kainate receptor signaling in neuropsychiatric disorders. For example, GluK2 ko mice exhibit behaviors analogous to mania, including increased risk taking, increased aggression, and less despair–like behavior (Shaltiel et al., 2008). GluK4 ko mice show reduced anxiety relative to their wildtype counterparts as well as a reduction in learned helplessness and increase in hedonic behavior (Catches et al., 2011). Conversely, increasing gene expression of Grik4 in the cortex and striatum increased anhedonic, anxiety, and depressive behaviors (Aller et al., 2015). These studies support human genetic studies that link variants in kainate receptor genes to bipolar disorder and schizophrenia (Knight et al., 2011; Pickard et al., 2008; Whalley et al., 2009). While these studies have been instructive in demonstrating a link to human disease, the dissection of the role of each of the subunits with a knockout approach has gone only part way to fully describing the roles of kainate receptors at synapses and their affects on behavior. This is a particular concern for kainate receptors, because native receptors are likely assembled from multiple subunits expressed in diverse pattern that changes over the course of development (Bahn et al., 1994; Wisden and Seeburg, 1993), making it uncertain whether all kainate receptor signaling is disrupted throughout the life of the animal in any particular brain region in the single subunit knockout studies. Additionally kainate receptors have been proposed as therapeutic targets for a number of neurological disorders (Lerma and Marques, 2013; Yuan et al., 2015), yet the lack of available pharmacology has not allowed investigation of a pan-kainate receptor blocker on cellular, circuit or behavioral function. To address these potential confounds we generated mice that lack expression of all five subunits of the kainate receptor (5ko mice). To our knowledge this is the first report of any mouse with a disruption of a complete gene family of ionotropic glutamate receptors.
We were surprised to find that this approach uncovered a strong emergent behavioral alteration in striatal-dependent preservative behavior and motor function. The 5ko mice have a striking phenotype involving self-injurious over-grooming and, consistent with striatal dysfunction, elevations in digging behavior and perseveration in a Y maze choice test (Burguiere et al., 2015). The mice also had multiple alterations in motor behaviors with a classic hind limb clasping phenotype, impairments in the accelerating rotarod, and alterations in gait. Kainate receptors are expressed in striatal neurons, yet little is known about their contribution to synaptic transmission (Chergui et al., 2000; Vizcarra-Chacon et al., 2013). We found that corticostriatal synaptic transmission in the dorsal striatum was impaired, with changes in the NMDA:AMPA ratio, as well as reductions in the field potential response and lower mEPSC frequencies both suggesting the existence of fewer excitatory synapses. We did not detect a change in amplitude of mEPSCs suggesting that the quantal efficacy of individual synapses was not altered. Therefore, the simplest explanation for the reduced NMDA:AMPA ratio would be that the NMDA component of individual striatal synapses is reduced in 5ko mice. Alterations in the NMDA:AMPA ratio are observed in several animal models and could be a cellular correlate of repetitive grooming and perseverative behavior (Blundell et al., 2010). These data focus attention on the importance of kainate receptors to the development of striatal circuits and their prominent effect on disrupting striatal-dependent behaviors.
This study also raises the possibility of multiple different roles for kainate receptors in regulating striatal activity and the development of striatal circuits. GluK2-containing receptors are required for expression of ionotropic kainate receptor currents in SPNs of adult/juvenile mice (Chergui et al., 2000). However, the studies here demonstrate that loss of GluK2 alone is not sufficient to reproduce many of striatal dependent behavioral deficits seen in 5ko mice. This could indicate that compensation by other receptor subunits occurs in the single knockout or that other kainate receptor subunits play a role in early striatal development. In fact, there is evidence of a more diverse population of kainate receptor subunits expressed in the striatum in the embryo and during early postnatal development than is found in the adult (Bahn et al., 1994). However, approach of making constitutive deletion of all the kainate receptor genes throughout the life of the animal do not allow us to clarify whether kainate receptor loss during development are the primary cause of the phenotype, or whether ongoing kainate receptor signaling in adult mice is required for proper striatal function. Likewise another limitation of this approach is that while the data are strongly suggestive, we are not able to unequivocally determine that the synaptic disruptions are due to cell autonomous processes resulting from loss of kainate receptor signaling in SPNs. Approaches in the future such as conditional ablation of receptor genes at different developmental timepoints will be needed to clarify these issues.
5ko mice parallel characteristics of mouse models of compulsive disorders
The striatum forms the major input structure of the basal ganglia and SPNs integrate convergent information to control planning and modulation of movement. Human and animal studies show that striatal circuits are critical to perseverative and habitual behaviors, and alteration of synaptic transmission in this region underlies the pathophysiology of OCD (Burguiere et al., 2015). Recently, studies in knockout mice have demonstrated that loss of proteins specifically associated with excitatory synapses, such as the scaffolding molecules Sapap3 and Shank3, alter striatal glutamate signaling and cause OCD-like behavior, similar to that observed in 5ko mice. Sapap3 is enriched in the striatum and ablation of this molecule in mice results in over-grooming behavior and selective deficits in corticostriatal synaptic transmission (Welch et al., 2007). We found evidence that native kainate receptors associate with Sapap co-immunoprecipitating from striatal homogenates. Shank3 also interacts with Sapap and potentially exists in a complex with PSD95 and glutamate receptors. Shank3 ko mice display self-injurious grooming, reduced corticostriatal transmission and reduced spine density in SPNs similar to the behavioral and synaptic perturbations in 5ko mice. Given the overlapping phenotype between 5ko mice and these other mouse models of OCD and autism it will be important to assess kainate receptor genes in the context of these disorders. Indeed, there have been several studies suggesting that kainate receptors are candidate genes for OCD (Sampaio et al., 2010) and autism susceptibility (Delorme et al., 2004; Jamain et al., 2002). The emergent phenotype in 5ko mice further underlines the known association between kainate receptor genes and perseverative and autistic-like traits in human disorders.
Methods
Animals
Animals were group housed with 14-hr:10-hr Light/Dark cycle. Food and water were provided ad libitum. All experiments were approved by the Northwestern University IACUC.
Self-grooming manual scoring
Individual mice (4–6 months) were habituated to a new standard mouse cage with a 0.5 cm layer of bedding to reduce neophobia then video-taped for 30 minutes undisturbed. Cumulative time spent grooming for each mouse was scored with a stopwatch.
Automated grooming and movement
Mice (male 4–6 months) were individually housed in metabolic PhenoMaster chambers for six days on a 12hr:12hr lighting schedule. After 2 days of habituation automated acquisition of several parameters including fine movement counts and ambulatory locomoter activity were monitored continuously over the entire period.
Marble-burying test
24 dark marbles were placed on top of wood chip bedding (3 cm deep) in six rows in a cage placed in a brightly lit, open area. Animals remained in the testing cage for 15 mins. Mice were manually scored for digging time using a stopwatch. At the end of the test period, counts were made of the number of marbles buried by more than two-thirds of their diameter.
Spontaneous alternations in the Y - maze
Mice were tested over 6 days with 1 trial per day. Mice were placed in the starting gate for 30s, and allowed to explore the maze. Once the mouse made a choice between the two arms, the entrance to the arm was blocked and the mouse remained in the blocked arm for 30s. The mouse was immediately placed back at the start and allowed to explore the maze again. The choice of arm on the subsequent test was scored as either 1 (same arm) or 0 (alternate arm).
Hind limb clasping
Hind limb clasping analysis was carried out according to prior studies (Guyenet et al., 2010). Mice were lifted by the base of the tail and hind limb position observed for 10 seconds.
Rotarod test
Mice were placed on the rotarod and the initial revolution speed set to 4 rpm for 120 seconds. The rotarod them accelerated at a rate of 0.12 rpm s−1 for the next 300 seconds, Mice were subjected to three trials per day for 4 consecutive days with a ~30 mins inter-trial interval.
Gait Analysis
Automated measurements of mouse gait where measured as mice walked on a treadmill at constant velocity of 17 cm s−1. Swing and stride parameters were determined from a 5 s video.
Three chamber social interaction test
In phase 1 of the test, mice were introduced to the middle compartment (C) of a three chamber apparatus. Mice were allowed 6 minutes to freely explore left and right chambers each containing an empty wired cup (E1 & E2). In phase 2, a juvenile mouse of the same gender was placed in the wired cup in the left compartment (M). Test animals were allowed to freely navigate the apparatus and tracked by a video tracking system.
Western Blots and co-Immunoprecipitation
Synaptoneurosomes were prepared using similar procedures to those previously described (Antion et al., 2010). Cell culture, transfection, immunoprecipitation and Western blot analysis were performed as described previously (Gill et al., 2009). The following primary antibodies were used: Anti-PSD-95 (Affinity BioReagents, MA1-046), Anti-GluK2/3 antibody (Chemicon, AB5683), Anti-PAN-SAPAP (NeuroMab, N127/31).
Slice procedure and Electrophysiological Recording
Parasagittal or coronal slices containing the dorsal striatum or horizontal hippocampal slices were prepared using techniques modified from those previously described (Xu et al., 2014). Individual slices were transferred to a recording chamber and visualized. Extracellular striatal field responses were evoked using a monopolar electrode filled with ACSF placed in the white matter between the cortex and the striatum. For whole-cell experiments, SPNs were visually identified and were voltage clamped at −70 mV for measurement of AMPA current (peak) and +40 mV for measurement of NMDA currents (measured in a 2.5ms window, 60ms after the onset of the current) in the presence of the GABAA receptor antagonist bicuculline (10μM). Kainate mediated currents were isolated by the addition of the AMPA receptor antagonist GYKI53655 (50μM) and NMDA receptor antagonist D-APV (50μM). Miniature events (mEPSCs) were recorded in the presence of TTX (1μM), D-APV (50μM) and bicuculline (10 μM).
Single cell RT-PCR
For single cell gene profiling the intracellular contents of the recorded cells were harvested in the patch electrode after completion of the experiment. Reverse transcription was performed using SuperScript VILO kit (Invitrogen). cDNA was amplified by PCR using substance P, enkephalin, and β-actin primer pairs. D1 neurons were characterized by the presence of a substance P band, a β-actin band and the absence of the enkephalin band. D2 cells were characterized by presence of enkephalin band, β-actin band and the absence of the substance P band.
Two Photon Laser Scanning Microscopy
Patch clamp recordings were made from visually identified spiny projection neurons including AlexaFluor 568 dye (50 μM) in the internal solution. Fluorescent images were acquired with picosecond pulsed excitation at 790 nM. Maximum projection images of the soma and dendritic field were acquired with 0.19 μm2 pixels with 15.2 μs pixel dwell time. Approximately 60 images were taken with 0.7 μm focal steps. Images were deconvolved using the Iterative Deconvolve 3D plugin for ImageJ and semi-automated spine counting was performed using three-dimensional reconstructions in NeuronStudio.
Data analysis
Statistical analyses were conducted with Microsoft Excel, Graphpad Prism, OriginPro9.0, and MATLAB software. For continuously distributed data, two sample comparisons were made using an unpaired two-tailed Student’s t-test or for multiple comparisons a repeated two-way analysis of variance (ANOVA) followed by post-hoc Sidak’s correction was performed. For ordinal data a Kruskall-Wallis test followed by a Mann-Whitney U-test was used where indicated. Differences were considered significant when p < 0.05. Data are shown as mean ± SEM.
Supplementary Material
Acknowledgments
This work was funded by grants from NIH/NIMH (R01MH099114), NIH/NINDS (R21NS082785), and the McKnight Foundation (to AC), and NIH/NINDS (R01NS071952) to GTS. JJM was supported by a fellowship from NIH/NIMH (1F31MH099807) and JX was supported by a grant from NIH/NIMH (K01MH094464). We thank Mr. Stephen Kraniotis for technical support, Mr. James Paik for help with the Y-maze, and Dr Kathryn Alpert for help with MRI analysis. We also acknowledge Prof Stephen Heinemann for providing mice.
Footnotes
Author Contributions: JX, JJM, HF, TN and YZ generated mice and performed behavioral, anatomical, biochemical and cellular physiology experiments. BAC performed biochemical experiments. DP, SM and LM generated and analyzed MRI data. JX, GTS and AC wrote the manuscript, which was edited by all authors. JX, JJM and AC conceived and designed the initial experiments.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Citations
- Ahmari SE, Spellman T, Douglass NL, Kheirbek MA, Simpson HB, Deisseroth K, Gordon JA, Hen R. Repeated cortico-striatal stimulation generates persistent OCD-like behavior. Science. 2013;340:1234–1239. doi: 10.1126/science.1234733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aller MI, Pecoraro V, Paternain AV, Canals S, Lerma J. Increased Dosage of High-Affinity Kainate Receptor Gene grik4 Alters Synaptic Transmission and Reproduces Autism Spectrum Disorders Features. J Neurosci. 2015;35:13619–13628. doi: 10.1523/JNEUROSCI.2217-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antion MD, Christie LA, Bond AM, Dalva MB, Contractor A. Ephrin-B3 regulates glutamate receptor signaling at hippocampal synapses. Mol Cell Neurosci. 2010 doi: 10.1016/j.mcn.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahn S, Volk B, Wisden W. Kainate receptor gene expression in the developing rat brain. J Neurosci. 1994;14:5525–5547. doi: 10.1523/JNEUROSCI.14-09-05525.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begni S, Popoli M, Moraschi S, Bignotti S, Tura GB, Gennarelli M. Association between the ionotropic glutamate receptor kainate 3 (GRIK3) ser310ala polymorphism and schizophrenia. Mol Psychiatry. 2002;7:416–418. doi: 10.1038/sj.mp.4000987. [DOI] [PubMed] [Google Scholar]
- Blundell J, Blaiss CA, Etherton MR, Espinosa F, Tabuchi K, Walz C, Bolliger MF, Sudhof TC, Powell CM. Neuroligin-1 deletion results in impaired spatial memory and increased repetitive behavior. J Neurosci. 2010;30:2115–2129. doi: 10.1523/JNEUROSCI.4517-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burguiere E, Monteiro P, Mallet L, Feng G, Graybiel AM. Striatal circuits, habits, and implications for obsessive-compulsive disorder. Curr Opin Neurobiol. 2015;30:59–65. doi: 10.1016/j.conb.2014.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catches JS, Xu J, Contractor A. Genetic ablation of the GluK4 kainate receptor subunit causes anxiolytic and antidepressant-like behavior in mice. Behav Brain Res. 2011 doi: 10.1016/j.bbr.2011.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chergui K, Bouron A, Normand E, Mulle C. Functional GluR6 kainate receptors in the striatum: indirect downregulation of synaptic transmission. J Neurosci. 2000;20:2175–2182. doi: 10.1523/JNEUROSCI.20-06-02175.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contractor A, Mulle C, Swanson GT. Kainate receptors coming of age: milestones of two decades of research. Trends Neurosci. 2011;34:154–163. doi: 10.1016/j.tins.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contractor A, Sailer AW, Darstein M, Maron C, Xu J, Swanson GT, Heinemann SF. Loss of kainate receptor-mediated heterosynaptic facilitation of mossy-fiber synapses in KA2−/− mice. J Neurosci. 2003;23:422–429. doi: 10.1523/JNEUROSCI.23-02-00422.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme R, Krebs MO, Chabane N, Roy I, Millet B, Mouren-Simeoni MC, Maier W, Bourgeron T, Leboyer M. Frequency and transmission of glutamate receptors GRIK2 and GRIK3 polymorphisms in patients with obsessive compulsive disorder. Neuroreport. 2004;15:699–702. doi: 10.1097/00001756-200403220-00025. [DOI] [PubMed] [Google Scholar]
- Fernandes HB, Catches JS, Petralia RS, Copits BA, Xu J, Russell TA, Swanson GT, Contractor A. High-affinity kainate receptor subunits are necessary for ionotropic but not metabotropic signaling. Neuron. 2009;63:818–829. doi: 10.1016/j.neuron.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia EP, Mehta S, Blair LA, Wells DG, Shang J, Fukushima T, Fallon JR, Garner CC, Marshall J. SAP90 binds and clusters kainate receptors causing incomplete desensitization. Neuron. 1998;21:727–739. doi: 10.1016/s0896-6273(00)80590-5. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Surmeier DJ. Modulation of striatal projection systems by dopamine. Annu Rev Neurosci. 2011;34:441–466. doi: 10.1146/annurev-neuro-061010-113641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill MB, Vivithanaporn P, Swanson GT. Glutamate binding and conformational flexibility of ligand-binding domains are critical early determinants of efficient kainate receptor biogenesis. J Biol Chem. 2009;284:14503–14512. doi: 10.1074/jbc.M900510200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet SJ, Furrer SA, Damian VM, Baughan TD, La Spada AR, Garden GA. A simple composite phenotype scoring system for evaluating mouse models of cerebellar ataxia. J Vis Exp. 2010 doi: 10.3791/1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamain S, Betancur C, Quach H, Philippe A, Fellous M, Giros B, Gillberg C, Leboyer M, Bourgeron T. Linkage and association of the glutamate receptor 6 gene with autism. Mol Psychiatry. 2002;7:302–310. doi: 10.1038/sj.mp.4000979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight HM, Walker R, James R, Porteous DJ, Muir WJ, Blackwood DH, Pickard BS. GRIK4/KA1 protein expression in human brain and correlation with bipolar disorder risk variant status. Am J Med Genet B Neuropsychiatr Genet. 2011 doi: 10.1002/ajmg.b.31248. [DOI] [PubMed] [Google Scholar]
- Lerma J, Marques JM. Kainate receptors in health and disease. Neuron. 2013;80:292–311. doi: 10.1016/j.neuron.2013.09.045. [DOI] [PubMed] [Google Scholar]
- Mattheisen M, Samuels JF, Wang Y, Greenberg BD, Fyer AJ, McCracken JT, Geller DA, Murphy DL, Knowles JA, Grados MA, et al. Genome-wide association study in obsessive-compulsive disorder: results from the OCGAS. Mol Psychiatry. 2015;20:337–344. doi: 10.1038/mp.2014.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei Y, Monteiro P, Zhou Y, Kim JA, Gao X, Fu Z, Feng G. Adult restoration of Shank3 expression rescues selective autistic-like phenotypes. Nature. 2016;530:481–484. doi: 10.1038/nature16971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motazacker MM, Rost BR, Hucho T, Garshasbi M, Kahrizi K, Ullmann R, Abedini SS, Nieh SE, Amini SH, Goswami C, et al. A defect in the ionotropic glutamate receptor 6 gene (GRIK2) is associated with autosomal recessive mental retardation. Am J Hum Genet. 2007;81:792–798. doi: 10.1086/521275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulle C, Sailer A, Perez-Otano I, Dickinson-Anson H, Castillo PE, Bureau I, Maron C, Gage FH, Mann JR, Bettler B, et al. Altered synaptic physiology and reduced susceptibility to kainate-induced seizures in GluR6-deficient mice. Nature. 1998;392:601–605. doi: 10.1038/33408. [DOI] [PubMed] [Google Scholar]
- Mulle C, Sailer A, Swanson GT, Brana C, O’Gorman S, Bettler B, Heinemann SF. Subunit composition of kainate receptors in hippocampal interneurons. Neuron. 2000;28:475–484. doi: 10.1016/s0896-6273(00)00126-4. [DOI] [PubMed] [Google Scholar]
- Peca J, Feliciano C, Ting JT, Wang W, Wells MF, Venkatraman TN, Lascola CD, Fu Z, Feng G. Shank3 mutant mice display autistic-like behaviours and striatal dysfunction. Nature. 2011;472:437–442. doi: 10.1038/nature09965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickard BS, Knight HM, Hamilton RS, Soares DC, Walker R, Boyd JK, Machell J, Maclean A, McGhee KA, Condie A, et al. A common variant in the 3′UTR of the GRIK4 glutamate receptor gene affects transcript abundance and protects against bipolar disorder. Proc Natl Acad Sci U S A. 2008;105:14940–14945. doi: 10.1073/pnas.0800643105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickard BS, Malloy MP, Christoforou A, Thomson PA, Evans KL, Morris SW, Hampson M, Porteous DJ, Blackwood DH, Muir WJ. Cytogenetic and genetic evidence supports a role for the kainate-type glutamate receptor gene, GRIK4, in schizophrenia and bipolar disorder. Mol Psychiatry. 2006;11:847–857. doi: 10.1038/sj.mp.4001867. [DOI] [PubMed] [Google Scholar]
- Pinheiro PS, Mulle C. Presynaptic glutamate receptors: physiological functions and mechanisms of action. Nat Rev Neurosci. 2008;9:423–436. doi: 10.1038/nrn2379. [DOI] [PubMed] [Google Scholar]
- Sampaio AS, Fagerness J, Crane J, Leboyer M, Delorme R, Pauls DL, Stewart SE. Association Between Polymorphisms in GRIK2 Gene and Obsessive-Compulsive Disorder: A Family-Based Study. CNS Neurosci Ther. 2010 doi: 10.1111/j.1755-5949.2009.00130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaltiel G, Maeng S, Malkesman O, Pearson B, Schloesser RJ, Tragon T, Rogawski M, Gasior M, Luckenbaugh D, Chen G, et al. Evidence for the involvement of the kainate receptor subunit GluR6 (GRIK2) in mediating behavioral displays related to behavioral symptoms of mania. Mol Psychiatry. 2008;13:858–872. doi: 10.1038/mp.2008.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmelkov SV, Hormigo A, Jing D, Proenca CC, Bath KG, Milde T, Shmelkov E, Kushner JS, Baljevic M, Dincheva I, et al. Slitrk5 deficiency impairs corticostriatal circuitry and leads to obsessive-compulsive-like behaviors in mice. Nat Med. 2010;16:598–602. doi: 10.1038/nm.2125. 591p following 602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub C, Noam Y, Nomura T, Yamasaki M, Yan D, Fernandes HB, Zhang P, Howe JR, Watanabe M, Contractor A, et al. Distinct Subunit Domains Govern Synaptic Stability and Specificity of the Kainate Receptor. Cell Rep. 2016;16:531–544. doi: 10.1016/j.celrep.2016.05.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas A, Burant A, Bui N, Graham D, Yuva-Paylor LA, Paylor R. Marble burying reflects a repetitive and perseverative behavior more than novelty-induced anxiety. Psychopharmacology (Berl) 2009;204:361–373. doi: 10.1007/s00213-009-1466-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vizcarra-Chacon BJ, Arias-Garcia MA, Perez-Ramirez MB, Flores-Barrera E, Tapia D, Drucker-Colin R, Bargas J, Galarraga E. Contribution of different classes of glutamate receptors in the corticostriatal polysynaptic responses from striatal direct and indirect projection neurons. BMC Neurosci. 2013;14:60. doi: 10.1186/1471-2202-14-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch JM, Lu J, Rodriguiz RM, Trotta NC, Peca J, Ding JD, Feliciano C, Chen M, Adams JP, Luo J, et al. Cortico-striatal synaptic defects and OCD-like behaviours in Sapap3-mutant mice. Nature. 2007;448:894–900. doi: 10.1038/nature06104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalley HC, Pickard BS, McIntosh AM, Zuliani R, Johnstone EC, Blackwood DH, Lawrie SM, Muir WJ, Hall J. A GRIK4 variant conferring protection against bipolar disorder modulates hippocampal function. Mol Psychiatry. 2009;14:467–468. doi: 10.1038/mp.2009.7. [DOI] [PubMed] [Google Scholar]
- Wisden W, Seeburg PH. A complex mosaic of high-affinity kainate receptors in rat brain. J Neurosci. 1993;13:3582–3598. doi: 10.1523/JNEUROSCI.13-08-03582.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright DJ, Renoir T, Smith ZM, Frazier AE, Francis PS, Thorburn DR, McGee SL, Hannan AJ, Gray LJ. N-Acetylcysteine improves mitochondrial function and ameliorates behavioral deficits in the R6/1 mouse model of Huntington’s disease. Transl Psychiatry. 2015;5:e492. doi: 10.1038/tp.2014.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Antion MD, Nomura T, Kraniotis S, Zhu Y, Contractor A. Hippocampal metaplasticity is required for the formation of temporal associative memories. J Neurosci. 2014;34:16762–16773. doi: 10.1523/JNEUROSCI.2869-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadin E, Friedman E, Bridger WH. Spontaneous alternation behavior: an animal model for obsessive-compulsive disorder? Pharmacol Biochem Behav. 1991;40:311–315. doi: 10.1016/0091-3057(91)90559-k. [DOI] [PubMed] [Google Scholar]
- Yuan H, Low CM, Moody OA, Jenkins A, Traynelis SF. Ionotropic GABA and Glutamate Receptor Mutations and Human Neurologic Diseases. Mol Pharmacol. 2015;88:203–217. doi: 10.1124/mol.115.097998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Saur T, Duke AN, Grant SG, Platt DM, Rowlett JK, Isacson O, Yao WD. Motor impairments, striatal degeneration, and altered dopamine-glutamate interplay in mice lacking PSD-95. J Neurogenet. 2014;28:98–111. doi: 10.3109/01677063.2014.892486. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.