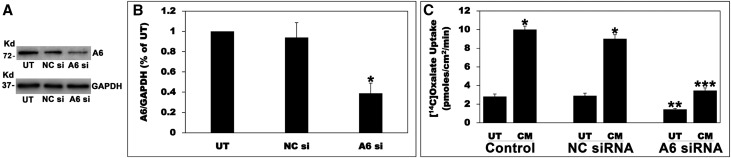
Figure 10.
Silencing A6 greatly reduced the CM-induced stimulation of oxalate transport. (A) A representative Western blot analysis of total A6 protein expression. A6 protein expression was evaluated in C2 cell lysate (10 µg protein/lane: untransfected cells; NC si, C2 cells transfected with the NC si; A6 si, C2 cells transfected with the siRNA targeting A6). The lower half of the same blot was probed with an anti-GAPDH antibody to normalize loading of protein in each lane (lower panel). (B) Densitometry of immunoblot results. Western blot band density was quantified using ImageJ software. Values are means±SEM for six independent experiments of relative total A6 abundance to GAPDH and are presented as a percentage of the UT value. A6 siRNA knockdown significantly reduced A6 protein expression (*P<0.001 for A6 si compared with UT and NC si, by ANOVA). (C) Effect of A6 silencing on the CM-induced stimulation of 14C-oxalate uptake by C2 cells. Untransfected (Control), NC siRNA (transfected with the NC si), and A6 siRNA (transfected with the siRNA targeting A6) C2 cells were UT or were treated apically with the CM in the culture medium (1:50 dilution × 24 hours), and then 14C-oxalate uptake was measured as described in the Concise Methods. Values are means±SEM of eight independent experiments each of which was done in triplicate. Silencing A6 significantly reduced the CM-induced stimulation of oxalate transport by C2 cells (*P<0.001 for CM compared with UT in the Control and NC siRNA; **P<0.01 for UT in A6 siRNA compared with UT in Control and NC siRNA; ***P<0.001 for CM in A6 siRNA compared with CM in Control and NC siRNA, by ANOVA).