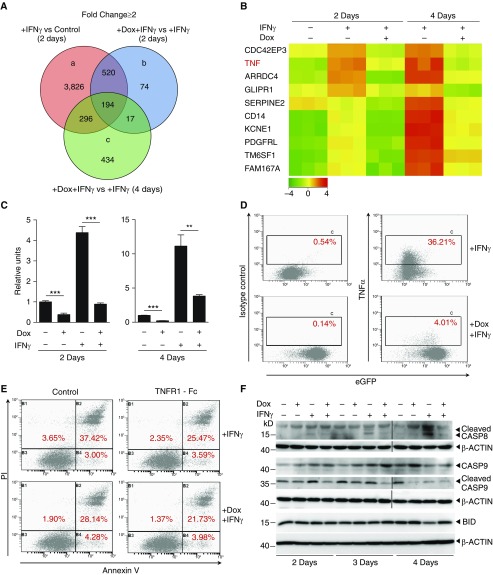
Figure 2.
SP110b expression downregulates tumor necrosis factor-α (TNF-α) production, resulting in suppression of IFN-γ–induced cell death. (A) Venn diagram showing the number of genes that were up- or downregulated following doxycycline (Dox)-induced SP110b expression in a THP1-enhanced green fluorescent protein (eGFP)-SP110b clonal cell line following microarray analyses at 2 and 4 days after IFN-γ treatment. (B) Heat map representing the set of differentially expressed genes regulated by SP110b in the THP1-eGFP-SP110b clonal cell line at 2 and 4 days post–IFN-γ treatment. −4 to 4 refer to log2 ratio values (log2 fold change). Orange indicates upregulated genes, and green indicates downregulated genes. (C and D) SP110b-mediated suppression of TNF-α expression in the THP1-eGFP-SP110b clonal cell line was validated (C) at the RNA level by quantitative polymerase chain reaction 2 and 4 days after treatment and (D) at the protein level by intracellular staining 2 days after treatment. (E) THP1 cell death was measured using fluorescence-activated cell sorting analysis of annexin V/propidium iodide (PI)-stained cells after 3 days of culture in the presence of conditioned medium. THP1 cells were cultured in conditioned medium (in the presence or absence of TNF receptor 1 [TNFR1]-Fc) that was generated from THP1-eGFP-SP110b cells that had been treated for 2 days with either IFN-γ alone or IFN-γ + Dox. See also Figure E2E. (F) Caspase-8 (CASP8), CASP9, and BH3-interacting domain death agonist (BID) expression was characterized with Western blot analyses at the indicated time points post-treatment. Data in C are presented as the mean ± SD of three cultures. Statistical significance was calculated using a two-tailed, unpaired t test. **P < 0.01; ***P < 0.001. The results represent one experiment in A and B and three independent experiments in C–F.