ABSTRACT
Congenital cytomegalovirus (CMV) infection represents a relevant cause of deafness and neurological damage in newborns. Intrauterine CMV transmission might result after primary or nonprimary infections, though at different rates (30% versus 0.2%, respectively). At present, a prenatal diagnosis of CMV infection is based mainly on maternal serology, the detection of CMV-DNA in amniotic fluid and fetal blood, and ultrasound (US) and magnetic resonance imaging (MRI). Recent evidences suggest that congenital CMV infection may be an immune-mediated disease and that evaluation of humoral and especially T-cell immunities may improve the overall prenatal diagnosis. This review summarizes the most recent advancements in the diagnosis of maternal and prenatal CMV infections.
KEYWORDS: human cytomegalovirus, laboratory assays, congenital infections, IgG avidity, CMV-ELISPOT, CMV-QuantiFERON, immunoserology, imaging
INTRODUCTION
The human cytomegalovirus (CMV) is a ubiquitous betaherpesvirus causing morbidity and mortality in immunocompromised patients and congenitally infected fetuses and newborns, resulting in a broad range of disabilities, including sensorineural hearing loss (SNHL), visual impairment, and motor and cognitive deficits. Other transient symptoms of congenital CMV infection (cCMV) may include hepatosplenomegaly, thrombocytopenia, and jaundice. The global prevalence of cCMV has been estimated at 0.7% (1), and fetal CMV transmission may arise from a maternal primary or nonprimary infection. The highest rate of cCMV occurs after primary infections in seronegative mothers (30 to 40%), while nonprimary infections, including CMV reactivations or reinfections, result in cCMV in 0.2 to 2% of cases, suggesting that preconceptional immunity may play a role in preventing intrauterine transmission (2). If the main burden of congenital infections in Europe and North America results from primary infections, then nonprimary infections represent the main cause of cCMV in developing countries or in poor socioeconomical contexts, since the seroprevalences among the resident populations are much higher (3). The severities of infections in neonates and infants are highly variable. It has been estimated that 10 to 15% of congenitally infected neonates are symptomatic at birth, and 40 to 58% of them will experience permanent long-term sequelae. Moreover, 13.5% of children with asymptomatic infections will develop late-onset sequelae, mainly consisting of hearing impairments and neurologic deficits. The effectiveness of antiviral treatment during pregnancy is still debated. Most of the concerns are related to the potential genotoxicity and teratogenicity of the drugs. The clinical benefits of CMV-specific hyperimmune globulin treatment are also disputed, due to discordant results obtained from different studies (4, 5).
In the last decade, major efforts have been made to improve the early laboratory diagnosis of maternal and fetal infections. Despite these efforts, at present, many countries in the world have not yet adopted a consolidated CMV screening program for pregnant women. This review presents the currently available tools and strategies for diagnosing CMV infection in pregnancy and discusses the current drawbacks and future perspectives of prenatal cCMV diagnosis.
DIAGNOSIS OF MATERNAL CMV INFECTION
Clinical signs and symptoms in pregnant women.
In pregnant women, CMV infection is often pauci- or asymptomatic, and for this reason, it may be unnoticed clinically. Whenever present, the symptoms are generally nonspecific and resemble a mononucleosis- or flu-like syndrome, with fever, cervical lymphadenopathy, sore throat, fatigue, and myalgia. Laboratory findings may include lymphocytosis and elevated liver enzymes. Clinical symptoms are more likely to be present in primary-infected pregnant women than in women with recurrent infections or reactivations (6). Thereby, the diagnosis of maternal infection must take into account anamnestic data, including known or accidental CMV exposures, and clinical and laboratory data.
SEROLOGY
CMV IgG detection.
Serologic assays are the primary tools for assessing primary CMV infections during pregnancy. Table 1 summarizes the current uses, limitations, positive predictive values (PPVs), and negative predictive values (NPVs) in regard to fetal infections. At present, the gold standard for determining primary infections is CMV seroconversion, which is the detection of CMV IgG in a previously known nonimmune pregnant woman. However, since preconceptional serologic screening for CMV is not routinely performed in several countries, seroconversion data may not always be available. As a surrogate method, the detections of CMV IgM and low-avidity IgG have been proven to be effective for the serologic diagnosis of primary infections (7).
TABLE 1.
Methods for diagnosing cCMV
| Method | Use | Limitations | cCMV transmission [reference(s)] |
|
|---|---|---|---|---|
| PPV (%) | NPV (%) | |||
| Maternal serologya,b | ||||
| Anti-CMV IgM | Diagnosis of maternal CMV infection | Aspecific and crossreactive results described; CMV IgM may persist long after (re-)infection | 9.8–28.5 [51] | 100.0 [51] |
| Seroconversion | Diagnosis of maternal CMV infection | Baseline and follow-up samples are required | 25.0 [51] | 100.0 [51] |
| Anti-CMV IgG avidity | Discrimination between primary and nonprimary maternal CMV infection | Less informative after >18 week of gestation, cutoff thresholds vary among different assays | 18.8–26.3 [52] | 90.9–100.0 [52] |
| CMV DNA in maternal blooda,b | Diagnosis of active maternal CMV infection | Decreasing sensitivity after the first month postinfection | 56.3 [53] | 64.7 [53] |
| Amniocentesisb | ||||
| CMV culture | cCMV diagnosis | High turnaround time | 100.0 [6, 50] | 84.6–92.6 [6, 50] |
| CMV DNA | cCMV diagnosis | False-positive results described | 93.8–100.0 [6, 50] | 92.7–93.1 [6, 50] |
| Cordocentesisc | ||||
| CMV culture | cCMV diagnosis | High turnaround time | 100.0 [6] | 64.6 [6] |
| CMV DNA | cCMV diagnosis | None described | 100.0 [6, 50] | 84.4–94.4 [6, 50] |
| Anti-CMV IgM | cCMV diagnosis | Low sensitivity; only ≥20 weeks of gestation | 100.0 [6, 50] | 70.6–88.0 [6, 50] |
| Imaging examinations | ||||
| Ultrasound | Predict cCMV outcome and severity | Sensitivity dependent on the timing of examination; not specific for CMV; complex interpretation requiring highly specialized medical personnel/neuroradiology consulting | 22.2–70.6d [42, 54] | 88.3–95.7d [42, 54] |
| Magnetic resonance imaging | Predict cCMV outcome and severity | Sensitivity dependent on the timing of examination; not specific for CMV; complex interpretation requiring highly specialized medical personnel/neuroradiology consulting | 19.2–66.7d [42, 54] | 79.5–100.0d [42, 54] |
Limited correlations with cCMV.
Not associated with cCMV severity.
Higher risk of fetal damage/loss compared to amniocentesis.
Percentages for symptomatic cCMV in newborns.
CMV IgM detection.
CMV IgM antibodies are present during primary and nonprimary infections, and thus, are not really informative for determining seroconversion. CMV IgM might persist up to 6 to 9 months after infection, and false-positive results may be due to aberrant and nonspecific crossreactive IgM (mostly from herpes simplex virus [HSV], varicella-zoster virus [VZV], and Epstein-Barr virus [EBV] infections) or to the interference from rheumatoid factor or other autoimmune disorders. The majority of the commercial serologic tests are based on enzyme-linked immunosorbent assays (ELISAs) that detect antibody responses to viral lysates covering a large repertoire of CMV epitopes. Studies comparing the performances of CMV IgM immunoenzymatic tests using native versus recombinant antigens revealed that the latter had lower sensitivities and specificities, probably due to antigen misfolding by a prokaryotic expression system (8). Interestingly, Busse et al. observed that the highest specificity was achieved with the Genzyme Virotech CMV ELISA, which combines crude viral lysates and the recombinant immediate-early protein 1 (IE1) (9).
Several semi- or fully automated commercial platforms assessing both CMV IgM and IgG are available and enable the processing and analyses of large batches of samples, while reducing potential operator-induced errors. The main methods include ELISAs, microparticle enzyme immunoassays (MEIAs), and chemiluminescent microparticle immunoassays (CMIAs). Comparisons among these systems highlighted excellent agreement rates (≥93%), but suboptimal correlations for IgM detection (55 to 79%, depending on the platform) (10). These discrepancies may be due to the extraordinary high sensitivities of some systems, which may detect extremely low levels of IgM that were either produced during CMV reactivation or persisted long after the acute infection (6). For the above considerations, it is recommended that any positive CMV IgM result be confirmed by different tests and interpreted only in the context of a complete serology panel for CMV (IgM, IgG, and IgG avidity index) (6, 10).
CMV IgG avidity determination.
The CMV IgG avidity assay is considered a primary tool to date the timing of an infection. This test is based on the notion that IgG avidity increases with time; low-avidity IgGs are associated with recent infections, while a high avidity index indicates past infections. The avidity is easily determined by coincubating the patient's serum with and without 6 M urea as a dissociating agent in an immunoenzymatic assay. The most important limitations of the CMV IgG avidity assays are the following. (i) There is variability in the ranges of low- and high-avidity thresholds among the different commercial kits available (11). (ii) The timing of the assay execution may critically affect the NPV. In fact, while a high IgG avidity index detected in the first trimester properly identifies past infections (NPV, 100%), intermediate-to-high values obtained after 21 weeks of pregnancy cannot rule out a primary infection (NPV, 90.9%) (6). (iii) The reported unusually long persistence (>18 weeks) of low-avidity CMV IgGs may potentially result in the misdiagnosis of a primary CMV infection, particularly when CMV IgMs are also detectable in serum (12). The reason for the latter phenomenon is still unclear. Studies evaluating the kinetics of CMV IgG avidity maturation in primary-infected pregnant women found different patterns within the same cohort analyzed; patients who displayed a rapid increase in the IgG avidity index had a higher risk of vertical CMV transmission (13). Thus, there is evidence that the duration and intensity of CMV viremia may directly affect the kinetics of IgG avidity maturation. This hypothesis was corroborated by Fornara and colleagues (14) who compared IgG titers and neutralizing antibodies from symptomatic, paucisymptomatic, and asymptomatic pregnant and nonpregnant patients. Remarkably, the authors also observed that anti-gB IgGs were the first-produced antibodies after a primary infection, followed by neutralizing antibodies and lastly by gH/gL/pUL128/130/131 and gH/gL IgG antibodies.
EPITOPE-SPECIFIC IgG IMMUNOBLOTS TO ASSESS PRIMARY VERSUS NONPRIMARY INFECTIONS
Epitope-specific IgG immunoblotting was investigated by Eggers et al. (15) to explore the potentially different patterns in primary versus nonprimary infections. The authors compared an in-house-developed anti-gB/gH epitope immunoblot assay to a standard microneutralization method. The authors showed that the CMV neutralizing and anti-gB/gH antibodies appeared 10 to 17 weeks after an infection, suggesting that gB/gH-specific immunoblotting may represent a useful bioassay to distinguish primary from nonprimary infections. This in-house-built Western blot presented a 93.6% sensitivity and 100% specificity for nonprimary infections. Moreover, Eggers et al. reported that a delayed anti-gB IgG appearance in primary infections was associated with a lower risk of intrauterine CMV transmission. Enders and colleagues (16) corroborated the immunoblot assay performances, suggesting its validity in determining the timing of CMV infection. Despite the diagnostic usefulness shown in these two reports, epitope-specific immunoblot assays are not commonly employed for CMV serologic diagnosis, since the assay is not standardized, is time-consuming, and requires complex data interpretation.
Nonprimary infections: reinfection or reactivation?
Boppana and colleagues hypothesized that reinfections with a different CMV strain may lead to a higher risk of cCMV compared with that from viral reactivations (17). In this scenario, the serologic evaluation of the anti-gB/gH IgG polymorphic regions response was proposed as a potential system to distinguish whether the maternal infection arose from different CMV strains or from CMV reactivation (18). In that study, the authors showed that 18/96 serum samples tested, which were obtained from CMV-seropositive women, had positive results for at least 2 CMV antigenic epitopes, suggesting that serologic analysis may distinguish CMV reactivation from coinfection and reinfection. One important caveat of this system is the requirement for a prior patient serology specimen to use as a reference. The evaluation of the serologic response to polymorphic CMV epitopes would have promising applications in the assessment of cCMV risk, particularly in the populations characterized by a high CMV seroprevalence, where recurrent infections are more likely to occur.
DETECTION OF CMV IN BIOLOGICAL FLUIDS
Molecular methods comprise the most common and standardized systems for detecting CMV nucleic acids to diagnose active CMV replication. Alternatively, direct viral isolation in cell culture provides the biological evidence for actively replicating virus.
Biological specimens required for CMV diagnosis.
CMV can be isolated from several biological samples, including blood, urine, saliva, semen, vaginal secretions, and amniotic fluid. After collecting, the samples should be stored at 4°C and immediately sent to the laboratory for testing, especially when requesting CMV isolation from cell culture. Even though CMV is excreted in multiple biological fluids, blood and urine are the most commonly used specimens for testing. CMV shedding in urine is known to persist intermittently for a long time after a primary infection; thus, urine testing increases the chances of detecting an ongoing infection. The detection of virus in blood is mostly found in primary-infected pregnant women (19). Viremia usually persists for one month after a primary infection; however, it has also been reported in seropositive women with recurrent infections, though at a lower rate (20, 21).
Direct isolation of CMV.
Viral isolation in cell culture is still considered the gold standard for diagnosing CMV infection, since it provides evidence of actively replicating virus. Urine specimens are very often used for isolating CMV in cell culture, whereas CMV isolation from peripheral blood is rarely performed, given the low sensitivity of the assay (6, 21). Primary human fibroblasts, mink lung epithelial cells (Mv1Lu), MRC-5 fibroblasts, and more recently, R-mix cells are permissive for CMV entry and replication and can be used for CMV diagnosis (22). The first and most relevant limitation of the viral culture method is the turnaround time required for viral replication. In the presence of a high viral load, CMV foci may be evident 24 h after inoculating, but whenever the viral load is low, the CMV cytopathic effect may be visible only after 10 to 30 days. The second most important limitation related with viral culture is that contaminant microbial flora in the biological specimens may lead to cell culture contamination and toxicity. Other limitations of the culture assay are the standardization and interpretation of microscopic morphologies.
Rapid isolation of CMV by the shell vial method has been proposed as an alternative to standard cell culture and enables the detection of CMV within 24 h. The method requires direct and indirect immunofluorescence assays detecting the CMV immediate early IE1/IE2 proteins, which correlate with the presence of actively replicating virus. Studies performed over 21 to 30 days showed a good correlation between rapid and standard cell culture isolations, although sample cytotoxicity, defective and aberrant CMV particles, and low viral titers may affect the assay performances (23, 24). Despite the fact that the shell vial method is considered more sensitive than standard culturing, one report suggests that both assays should be used to maximize CMV detection, especially when blood specimens are used (23).
Molecular methods for CMV diagnosis.
Molecular assays have revolutionized the diagnosis of CMV infection, overcoming almost completely the previous cited methods. Several systems are available, including quantitative assays (nucleic acid sequence-based amplification [NASBA], the hybrid capture assay, and PCR) and qualitative PCR, nowadays rarely employed (25). Real-time PCR is the most commonly used method for the molecular diagnosis of CMV infections, due to its excellent sensitivity and specificity and to the availability of commercial kits and automated platforms. Moreover, PCR performance is less affected by specimen transport and storage (19). An important limitation of quantitative PCR lies in the interassay and interlaboratory variability of copy number determination, which might impact patient management and clinical decisions. Recently, the WHO released a CMV international standard to overcome the interassay and interlaboratory issues with the aim of reaching an interlaboratory variability of <0.5 log10 UI/ml (26). However, the multicentric study conducted with the WHO CMV standard showed interlaboratory variability higher than the expected target of 0.5 log10 UI/ml, so the route to CMV harmonization and standardization still requires further improvements.
MATERNAL CMV-SPECIFIC T-CELL IMMUNITY AS A MARKER FOR INTRAUTERINE INFECTION
Interferon gamma release assays (IGRA) are widely used to detect patients' cell-mediated immunities (CMIs). Enzyme-linked immunosorbent spot (ELISPOT) and QuantiFERON (QFT) assays are the most standardized and employed platforms and were recently used to detect CMV-specific CMI in solid organ and allogeneic hematopoietic stem cell transplant recipients (27, 28). Although their performances for CMV CMI were very similar, interassay differences were found (29, 30). Recently, several studies assessed the role of CMV CMI in pregnant women. Bialas and colleagues showed that CD4+ T-cell depletion in infected pregnant rhesus macaques led to dismal and more severe outcomes compared with those of immunocompetent pregnant primates, thus suggesting a protective role of CD4+ T cells with respect to cCMV (31). A previous study of primary-infected pregnant women had already identified CMV-specific CD4+ T cells as key players in maternal CMV infection, and a low lymphoproliferative response associated significantly with an increased risk of congenital transmission (32). In a study of a cohort of 80 pregnant women with active CMV infections, our group observed that pregnant women with primary infections had significantly higher CMV T-cell immune responses compared with those with nonprimary infections. Furthermore, in primary-infected cases, the maternal CMV-specific T-cell immunity positively correlated with congenital transmission (33). Although the results from interferon (IFN)-γ ELISPOT and CMV-QFT assays were correlated, only the ELISPOT assay discriminated between transmitter and nontransmitter mothers (34, 35). In particular, a higher risk of cCMV was observed when maternal CMV T-cell responses were >185 spots/2 × 105 peripheral blood mononuclear cells (PBMCs). An association of CMI with cCMV was also reported in a recent study employing CMV-QFT. Eldar-Yedidia and colleagues showed that a CMV-QFT response normalized to the positive control was associated with an increased risk of congenital infection (36). Although the above-mentioned studies of Saldan and colleagues and that of Eldar-Yedidia and colleagues find that the maternal CMI is a predictor of vertical CMV transmission, these studies differ in the performances of CMV-QFT for assessing cCMV. Larger studies should be performed to assess whether CMV-QFT predicts vertical CMV infection.
CONGENITAL CMV INFECTION AS AN IMMUNE-MEDIATED DISEASE?
Serological and immunological studies found that cCMV occurred in the presence of high TH1 (IFN-γ ELISPOT) and low and delayed TH2 (CMV IgG avidity) maternal responses. These findings suggest that an imbalanced maternal TH1/TH2 immune response may lead to cCMV. An altered cytokines pattern was previously reported at the placental level in cCMV cases. In 2012, Hamilton et al. reported increased monocyte chemoattractant protein 1 (MCP-1) and tumor necrosis factor (TNF)-α levels in placentae from CMV-infected stillborn neonates and in placental histocultures following CMV infection (37). Recently, it was also reported that CMV infection of the maternal decidua led to higher expressions of IFN-γ and IFN-γ-induced protein 10 (IP-10) (38). Perturbation of the cytokine immune balance at the placental and decidual levels and the triggering of a strong TH1 immunity may, in fact, favor viral dissemination to the fetus and also reduce nutrient uptake and gas exchange across the placenta, an alteration that could ultimately lead to tissue hypoxia and explain the neurological abnormalities observed in symptomatic babies. We speculate that an altered placental cytokine pattern, along with an imbalanced T-cell response in peripheral blood, may lead to focal lesions at the placenta, allowing CMV crossing.
PRENATAL DIAGNOSIS OF cCMV
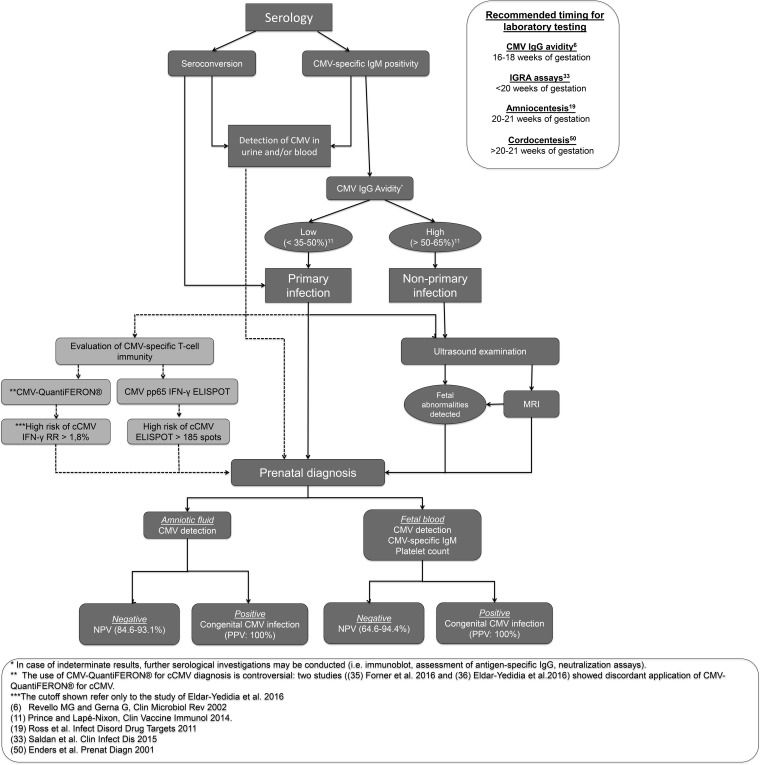
At present, invasive and noninvasive diagnoses of fetal infections can be offered to pregnant women with active CMV infection, particularly to primary-infected women at high risk of cCMV (Fig. 1). Invasive methods are based on amniocentesis and cordocentesis, while noninvasive methods include ultrasound (US) and magnetic resonance imaging (MRI).
FIG 1.
cCMV diagnosis flowchart. The novel findings on cCMV diagnosis are indicated in light gray. Relevant references for cCMV diagnosis are also reported.
Amniocentesis.
Prenatal diagnosis of cCMV can be accomplished either by viral culture or by detecting CMV DNA in an amniotic fluid specimen. Due to a higher sensitivity (90 to 100%), molecular diagnosis by PCR is presently preferred over CMV culture (19). As reported in Table 1, molecular methods show the highest PPVs (100%) for diagnosing fetal infections. However, the criteria for improving the NPV involve performing amniocentesis (i) 6 to 8 weeks after the estimated onset of maternal infection, and (ii) from 20 to 21 weeks of pregnancy onwards, when CMV excreted from fetal kidneys into the amniotic fluid becomes detectable. Even when following these indications, false-negative results were reported (6). Whether evaluating CMV viral loads in amniotic fluid predicts disease severity in newborns remains controversial. Some authors suggest that viral loads >105 genome equivalents (GEq)/ml are highly indicative of symptomatic disease, whereas values <103 GEq/ml exclude severe cCMV forms (19, 39, 40). Opposing results regarding the association between symptomatic disease and viral loads in amniotic fluid have been reported. Particularly, some authors found asymptomatic infants with viral loads >105 GEq/ml in the amniotic fluid and symptomatic infants with viral loads <105 GEq/ml (19). These discrepancies may result from differences in the timing for amniocentesis executions (19). For this reason, CMV DNA detection in amniotic fluid should consider not just the viral load by itself, but also the amniocentesis timing (40).
Cordocentesis.
Cordocentesis enables the evaluations of fetal blood infections, CMV IgM, and other hematological and biochemical parameters. Given that this invasive procedure carries a higher risk of adverse events compared with that from amniocentesis, its utility in the prenatal diagnosis of cCMV is still controversial. In fact, albeit showing 100% specificity, fetal CMV DNAemia and fetal serology have reduced sensitivities compared with that of amniocentesis (39). On the other hand, there is evidence that fetal CMV viral load and IgM titers tend to be higher in symptomatic fetuses (6), and more recently, thrombocytopenia was reported as a prognostic biomarker for cCMV severity (40). Given that cordocentesis does not improve the prenatal diagnosis of cCMV and it is potentially harmful to the fetus, the procedure should not be universally recommended, unless specific ethical and medical reasons suggest the execution of this approach.
CMV imaging in pregnancy.
Imaging tests during pregnancy can detect morphological fetal abnormalities compatible with cCMV, and may thus be useful both for the diagnosis of and prognosis for cCMV severity. Due to concerns for potential teratogenicity, computed tomography (CT) scans are not an option for assessing fetal development. Targeted US and MRI are the most commonly used systems for detecting fetal abnormalities. CMV infection may cause ventriculomegaly, periventricular echogenicity with or without cysts, intracranial calcifications, microcephaly, and cortical migrational abnormalities. Extracranial abnormalities can also be observed, mainly bowel hyperechogenicity, hepatosplenomegaly, and intrauterine growth retardation.
(i) Ultrasound imaging.
In the absence of a serologic screening program for CMV infection in pregnancy, US findings during the second or third trimester of pregnancy may provide the first evidence of presumptive cCMV. If the diagnosis of maternal and fetal infection by amniocentesis has already been established, regular US assessment every 3 to 5 weeks is recommended to detect signs of symptomatic fetal infection. Discordant results from several studies on US predictivity were reported for clinical outcomes of infected fetuses (41, 42), suggesting that the timing for US execution and transient fetal morphological features might critically influence the diagnosis. In fact, at present, only severe cranial abnormalities, such as ventriculomegaly and microcephaly, have been unequivocally associated with poor prognoses (43). Neurosonographic tests may improve the diagnostic performance, reaching PPV and NPV values comparable to those reported for MRI (44).
Recently, the combination of US features and fetal thrombocytopenia at the time of prenatal diagnosis showed 79% PPV and 91% NPV in predicting any symptoms at birth or at the termination of pregnancy, while US alone reached 93% NPV, similar to those of US and MRI executed during the third trimester (40 and reported in Table 1). The combined use of imaging and laboratory testing specifically pinpoints cCMV cases, since other common congenital infections (mainly toxoplasmosis, rubella, HSV, and VZV infections) and chromosomal disorders share similar neurological alterations indistinguishable by US alone (43).
(ii) MRI.
MRI is advised whenever fetal intracranial abnormalities are detected by US and should be performed during the third trimester (54). MRI was shown to be more sensitive than US (45), though results might be more difficult to interpret, and specialized neuroradiology consultation is required. It is generally accepted that negative MRI findings concomitant with negative US results reliably exclude severe outcomes for infected fetuses (46).
TO SCREEN OR NOT TO SCREEN: THE HAMLETIC DILEMMA AND FUTURE PERSPECTIVES
As previously stated, at present, a universal screening program for CMV infection in pregnant women has not been implemented worldwide for several reasons, including: (i) because safe and effective therapies and vaccines for preventing intrauterine transmission do not exist, (ii) because symptomatic cCMV might occur after primary or nonprimary maternal infection, and (iii) because only a limited proportion of congenitally infected newborns are symptomatic at birth or develop late-onset CMV-related sequelae. Several European countries (Austria, Belgium, France, Germany, Italy, the Netherlands, Portugal, and Spain) and Israel de facto offer serologic CMV screening and counseling to advise pregnant women on preventive strategies and eventual laboratory reevaluation (47). In other countries, such as in the United States, CMV testing is recommended only (i) when there is clinical suspicion of CMV infection, or (ii) fetal abnormalities are detected by routine imaging (48).
At present, there is a large debate on the costs for CMV screening. The estimated costs reported from U.S. studies refer only to the postnatal diagnosis and treatment of cCMV (49). Undoubtedly, any cost for maternal screening should consider not just the direct costs, but also the indirect future burden for disability management and detrimental social impact. Based on long-term experience, we have reasons to believe that maternal screening provides superior and beneficial results in both the short and long terms. However, relevant challenges need to be overcome in the near future to optimize maternal CMV screening, with a need to develop (i) more sensitive and specific assays to specifically pinpoint vertical transmission in nonprimary infections, (ii) novel noninvasive maternal biomarkers capable of assessing early cCMV transmission, and (iii) biomarkers predictive of the severity of fetal abnormalities.
ACKNOWLEDGMENT
This work was funded by a grant (no. 60A07-8071/14) from the University of Padua to D.A. Abate.
The authors declare no conflicts of interest.
REFERENCES
- 1.Dollard SC, Grosse SD, Ross DS. 2007. New estimates of the prevalence of neurological and sensory sequelae and mortality associated with congenital cytomegalovirus infection. Rev Med Virol 17:355–363. doi: 10.1002/rmv.544. [DOI] [PubMed] [Google Scholar]
- 2.Kenneson A, Cannon MJ. 2007. Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Rev Med Virol 17:253–276. doi: 10.1002/rmv.535. [DOI] [PubMed] [Google Scholar]
- 3.Mussi-Pinhata MM, Yamamoto AY, Moura Brito RM, de Lima Isaac M, de Carvalho e Oliveira PF, Boppana S, Britt WJ. 2009. Birth prevalence and natural history of congenital cytomegalovirus infection in a highly seroimmune population. Clin Infect Dis 49:522–528. doi: 10.1086/600882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nigro G, Adler SP, La Torre R, Best AM, Congenital Cytomegalovirus Collaborating Group. 2005. Passive immunization during pregnancy for congenital cytomegalovirus infection. N Engl J Med 353:1350–1362. doi: 10.1056/NEJMoa043337. [DOI] [PubMed] [Google Scholar]
- 5.Revello MG, Lazzarotto T, Guerra B, Spinillo A, Ferrazzi E, Kustermann A, Guaschino S, Vergani P, Todros T, Frusca T, Arossa A, Furione M, Rognoni V, Rizzo N, Gabrielli L, Klersy C, Gerna G, CHIP Study Group. 2014. A randomized trial of hyperimmune globulin to prevent congenital cytomegalovirus. N Engl J Med 370:1316–1326. doi: 10.1056/NEJMoa1310214. [DOI] [PubMed] [Google Scholar]
- 6.Revello MG, Gerna G. 2002. Diagnosis and management of human cytomegalovirus infection in the mother, fetus, and newborn infant. Clin Microbiol Rev 15:680–715. doi: 10.1128/CMR.15.4.680-715.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daiminger A, Bader U, Enders G. 2005. Pre- and periconceptional primary cytomegalovirus infection: risk of vertical transmission and congenital disease. BJOG 112:166–172. doi: 10.1111/j.1471-0528.2004.00328.x. [DOI] [PubMed] [Google Scholar]
- 8.BaAlawi F, Robertson PW, Lahra M, Rawlinson WD. 2012. Comparison of five CMV IgM immunoassays with CMV IgG avidity for diagnosis of primary CMV infection. Pathology 44:381–383. doi: 10.1097/PAT.0b013e328353bec0. [DOI] [PubMed] [Google Scholar]
- 9.Busse C, Strubel A, Schnitzler P. 2008. Combination of native and recombinant cytomegalovirus antigens in a new ELISA for detection of CMV-specific antibodies. J Clin Virol 43:137–141. doi: 10.1016/j.jcv.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 10.Lagrou K, Bodeus M, Van Ranst M, Goubau P. 2009. Evaluation of the new architect cytomegalovirus immunoglobulin M (IgM), IgG, and IgG avidity assays. J Clin Microbiol 47:1695–1699. doi: 10.1128/JCM.02172-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prince HE, Lape-Nixon M. 2014. Role of cytomegalovirus (CMV) IgG avidity testing in diagnosing primary CMV infection during pregnancy. Clin Vaccine Immunol 21:1377–1384. doi: 10.1128/CVI.00487-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lumley S, Patel M, Griffiths PD. 2014. The combination of specific IgM antibodies and IgG antibodies of low avidity does not always indicate primary infection with cytomegalovirus. J Med Virol 86:834–837. doi: 10.1002/jmv.23863. [DOI] [PubMed] [Google Scholar]
- 13.Ebina Y, Minematsu T, Morioka I, Deguchi M, Tairaku S, Tanimura K, Sonoyama A, Nagamata S, Morizane M, Yamada H. 2015. Rapid increase in the serum cytomegalovirus IgG avidity index in women with a congenitally infected fetus. J Clin Virol 66:44–47. doi: 10.1016/j.jcv.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 14.Fornara C, Furione M, Lilleri D, Cane I, Revello MG, Zavattoni M, Gerna G. 2015. Primary human cytomegalovirus infections: kinetics of ELISA-IgG and neutralizing antibody in pauci/asymptomatic pregnant women vs symptomatic non-pregnant subjects. J Clin Virol 64:45–51. doi: 10.1016/j.jcv.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 15.Eggers M, Radsak K, Enders G, Reschke M. 2001. Use of recombinant glycoprotein antigens gB and gH for diagnosis of primary human cytomegalovirus infection during pregnancy. J Med Virol 63:135–142. [PubMed] [Google Scholar]
- 16.Enders G, Daiminger A, Bader U, Exler S, Schimpf Y, Enders M. 2013. The value of CMV IgG avidity and immunoblot for timing the onset of primary CMV infection in pregnancy. J Clin Virol 56:102–107. doi: 10.1016/j.jcv.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 17.Boppana SB, Rivera LB, Fowler KB, Mach M, Britt WJ. 2001. Intrauterine transmission of cytomegalovirus to infants of women with preconceptional immunity. N Engl J Med 344:1366–1371. doi: 10.1056/NEJM200105033441804. [DOI] [PubMed] [Google Scholar]
- 18.Novak Z, Ross SA, Patro RK, Pati SK, Reddy MK, Purser M, Britt WJ, Boppana SB. 2009. Enzyme-linked immunosorbent assay method for detection of cytomegalovirus strain-specific antibody responses. Clin Vaccine Immunol 16:288–290. doi: 10.1128/CVI.00281-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ross SA, Novak Z, Pati S, Boppana SB. 2011. Overview of the diagnosis of cytomegalovirus infection. Infect Disord Drug Targets 11:466–474. doi: 10.2174/187152611797636703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arora N, Novak Z, Fowler KB, Boppana SB, Ross SA. 2010. Cytomegalovirus viruria and DNAemia in healthy seropositive women. J Infect Dis 202:1800–1803. doi: 10.1086/657412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Revello MG, Furione M, Rognoni V, Arossa A, Gerna G. 2014. Cytomegalovirus DNAemia in pregnant women. J Clin Virol 61:590–592. doi: 10.1016/j.jcv.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 22.Yang W, Hite S, Huang YT. 2005. Enhancement of cytomegalovirus detection in mink lung cells using CMV Turbo. J Clin Virol 34:125–128. doi: 10.1016/j.jcv.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 23.Gleaves CA, Smith TF, Shuster EA, Pearson GR. 1985. Comparison of standard tube and shell vial cell culture techniques for the detection of cytomegalovirus in clinical specimens. J Clin Microbiol 21:217–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rabella N, Drew WL. 1990. Comparison of conventional and shell vial cultures for detecting cytomegalovirus infection. J Clin Microbiol 28:806–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Atkinson C, Emery VC. 2011. Cytomegalovirus quantification: where to next in optimising patient management? J Clin Virol 51:223–228. doi: 10.1016/j.jcv.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 26.Preiksaitis JK, Hayden RT, Tong Y, Pang XL, Fryer JF, Heath AB, Cook L, Petrich AK, Yu B, Caliendo AM. 2016. Are we there yet? Impact of the first international standard for cytomegalovirus DNA on the harmonization of results reported on plasma samples. Clin Infect Dis 63:583–589. doi: 10.1093/cid/ciw370. [DOI] [PubMed] [Google Scholar]
- 27.Abate D, Fiscon M, Saldan A, Cofano S, Mengoli C, Sgarabotto D, d'Agostino C, Barzon L, Cusinato R, Toscano G, Feltrin G, Gambino A, Gerosa G, Palu G. 2012. Human cytomegalovirus-specific T-cell immune reconstitution in preemptively treated heart transplant recipients identifies subjects at critical risk for infection. J Clin Microbiol 50:1974–1980. doi: 10.1128/JCM.06406-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abate D, Cesaro S, Cofano S, Fiscon M, Saldan A, Varotto S, Mengoli C, Pillon M, Calore E, Biasolo MA, Cusinato R, Barzon L, Messina C, Carli M, Palu G. 2012. Diagnostic utility of human cytomegalovirus-specific T-cell response monitoring in predicting viremia in pediatric allogeneic stem-cell transplant patients. Transplantation 93:536–542. doi: 10.1097/TP.0b013e31824215db. [DOI] [PubMed] [Google Scholar]
- 29.Abate D, Saldan A, Mengoli C, Fiscon M, Silvestre C, Fallico L, Peracchi M, Furian L, Cusinato R, Bonfante L, Rossi B, Marchini F, Sgarabotto D, Rigotti P, Palu G. 2013. Comparison of cytomegalovirus (CMV) enzyme-linked immunosorbent spot and CMV quantiferon gamma interferon-releasing assays in assessing risk of CMV infection in kidney transplant recipients. J Clin Microbiol 51:2501–2507. doi: 10.1128/JCM.00563-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abate D, Saldan A, Forner G, Tinto D, Bianchin A, Palu G. 2014. Optimization of interferon gamma ELISPOT assay to detect human cytomegalovirus specific T-cell responses in solid organ transplants. J Virol Methods 196:157–162. doi: 10.1016/j.jviromet.2013.10.036. [DOI] [PubMed] [Google Scholar]
- 31.Bialas KM, Tanaka T, Tran D, Varner V, Cisneros De La Rosa E, Chiuppesi F, Wussow F, Kattenhorn L, Macri S, Kunz EL, Estroff JA, Kirchherr J, Yue Y, Fan Q, Lauck M, O'Connor DH, Hall AH, Xavier A, Diamond DJ, Barry PA, Kaur A, Permar SR. 2015. Maternal CD4+ T cells protect against severe congenital cytomegalovirus disease in a novel nonhuman primate model of placental cytomegalovirus transmission. Proc Natl Acad Sci U S A 112:13645–13650. doi: 10.1073/pnas.1511526112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Revello MG, Lilleri D, Zavattoni M, Furione M, Genini E, Comolli G, Gerna G. 2006. Lymphoproliferative response in primary human cytomegalovirus (HCMV) infection is delayed in HCMV transmitter mothers. J Infect Dis 193:269–276. doi: 10.1086/498872. [DOI] [PubMed] [Google Scholar]
- 33.Saldan A, Forner G, Mengoli C, Gussetti N, Palu G, Abate D. 2015. Strong cell-mediated immune response to human cytomegalovirus is associated with increased risk of fetal infection in primarily infected pregnant women. Clin Infect Dis 61:1228–1234. doi: 10.1093/cid/civ561. [DOI] [PubMed] [Google Scholar]
- 34.Saldan A, Forner G, Mengoli C, Tinto D, Fallico L, Peracchi M, Gussetti N, Palu G, Abate D. 2016. Comparison of the cytomegalovirus (CMV) enzyme-linked immunosorbent spot and CMV QuantiFERON cell-mediated immune assays in CMV-seropositive and -seronegative pregnant and nonpregnant women. J Clin Microbiol 54:1352–1356. doi: 10.1128/JCM.03128-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Forner G, Saldan A, Mengoli C, Gussetti N, Palu G, Abate D. 2016. Cytomegalovirus (CMV) enzyme-linked immunosorbent spot assay but not CMV QuantiFERON assay is a novel biomarker to determine risk of congenital CMV infection in pregnant women. J Clin Microbiol 54:2149–2154. doi: 10.1128/JCM.00561-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eldar-Yedidia Y, Bar-Meir M, Hillel M, Abitbol G, Broide E, Falk R, Assous M, Schlesinger Y. 2016. Low interferon relative-response to cytomegalovirus is associated with low likelihood of intrauterine transmission of the virus. PLoS One 11:e0147883. doi: 10.1371/journal.pone.0147883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hamilton ST, Scott G, Naing Z, Iwasenko J, Hall B, Graf N, Arbuckle S, Craig ME, Rawlinson WD. 2012. Human cytomegalovirus-induces cytokine changes in the placenta with implications for adverse pregnancy outcomes. PLoS One 7:e52899. doi: 10.1371/journal.pone.0052899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weisblum Y, Panet A, Zakay-Rones Z, Vitenshtein A, Haimov-Kochman R, Goldman-Wohl D, Oiknine-Djian E, Yamin R, Meir K, Amsalem H, Imbar T, Mandelboim O, Yagel S, Wolf DG. 2015. Human cytomegalovirus induces a distinct innate immune response in the maternal-fetal interface. Virology 485:289–296. doi: 10.1016/j.virol.2015.06.023. [DOI] [PubMed] [Google Scholar]
- 39.Lazzarotto T, Guerra B, Gabrielli L, Lanari M, Landini MP. 2011. Update on the prevention, diagnosis and management of cytomegalovirus infection during pregnancy. Clin Microbiol Infect 17:1285–1293. doi: 10.1111/j.1469-0691.2011.03564.x. [DOI] [PubMed] [Google Scholar]
- 40.Leruez-Ville M, Stirnemann J, Sellier Y, Guilleminot T, Dejean A, Magny JF, Couderc S, Jacquemard F, Ville Y. 2016. Feasibility of predicting the outcome of fetal infection with cytomegalovirus at the time of prenatal diagnosis. Am J Obstet Gynecol 215:342.e1–342.e9. doi: 10.1016/j.ajog.2016.03.052. [DOI] [PubMed] [Google Scholar]
- 41.Leyder M, Vorsselmans A, Done E, Van Berkel K, Faron G, Foulon I, Naessens A, Jansen A, Foulon W, Gucciardo L. 2016. Primary maternal cytomegalovirus infections: accuracy of fetal ultrasound for predicting sequelae in offspring. Am J Obstet Gynecol 215:638.e1–638.e8. doi: 10.1016/j.ajog.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 42.Benoist G, Salomon LJ, Mohlo M, Suarez B, Jacquemard F, Ville Y. 2008. Cytomegalovirus-related fetal brain lesions: comparison between targeted ultrasound examination and magnetic resonance imaging. Ultrasound Obstet Gynecol 32:900–905. doi: 10.1002/uog.6129. [DOI] [PubMed] [Google Scholar]
- 43.Malinger G, Lev D, Lerman-Sagie T. 2011. Imaging of fetal cytomegalovirus infection. Fetal Diagn Ther 29:117–126. doi: 10.1159/000321346. [DOI] [PubMed] [Google Scholar]
- 44.Malinger G, Ben-Sira L, Lev D, Ben-Aroya Z, Kidron D, Lerman-Sagie T. 2004. Fetal brain imaging: a comparison between magnetic resonance imaging and dedicated neurosonography. Ultrasound Obstet Gynecol 23:333–340. doi: 10.1002/uog.1016. [DOI] [PubMed] [Google Scholar]
- 45.Averill LW, Kandula VV, Akyol Y, Epelman M. 2015. Fetal brain magnetic resonance imaging findings in congenital cytomegalovirus infection with postnatal imaging correlation. Semin Ultrasound CT MR 36:476–486. doi: 10.1053/j.sult.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 46.Lipitz S, Hoffmann C, Feldman B, Tepperberg-Dikawa M, Schiff E, Weisz B. 2010. Value of prenatal ultrasound and magnetic resonance imaging in assessment of congenital primary cytomegalovirus infection. Ultrasound Obstet Gynecol 36:709–717. doi: 10.1002/uog.7657. [DOI] [PubMed] [Google Scholar]
- 47.Adler SP. 2011. Screening for cytomegalovirus during pregnancy. Infect Dis Obstet Gynecol 2011:1–9. doi: 10.1155/2011/942937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Centers for Disease Control and Prevention. 2008. Knowledge and practices of obstetricians and gynecologists regarding cytomegalovirus infection during pregnancy—United States, 2007. MMWR Morb Mortal Wkly Rep 57:65–68. [PubMed] [Google Scholar]
- 49.Rahav G. 2007. Congenital cytomegalovirus infection—a question of screening. Isr Med Assoc J 9:392–394. [PubMed] [Google Scholar]
- 50.Enders G, Bäder U, Lindemann L, Schalasta G, Daiminger A. 2001. Prenatal diagnosis of congenital cytomegalovirus infection in 189 pregnancies with known outcome. Prenat Diagn 21:362–377. doi: 10.1002/pd.59. [DOI] [PubMed] [Google Scholar]
- 51.Lazzarotto T, Guerra B, Spezzacatena P, Varani S, Gabrielli L, Pradelli P, Rumpianesi F, Banzi C, Bovicelli L, Landini MP. 1998. Prenatal diagnosis of congenital cytomegalovirus infection. J Clin Microbiol 36:3540–3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lazzarotto T, Spezzacatena P, Varani S, Gabrielli L, Pradelli P, Guerra B, Landini MP. 1999. Anticytomegalovirus (anti-CMV) immunoglobulin G avidity in identification of pregnant women at risk of transmitting congenital CMV infection. Clin Diagn Lab Immunol 6:127–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lazzarotto T, Gabrielli L, Lanari M, Guerra B, Bellucci T, Sassi M, Landini MP. 2004. Congenital cytomegalovirus infection: recent advances in the diagnosis of maternal infection. Hum Immunol 65:410–415. doi: 10.1016/j.humimm.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 54.Cannie MM, Devlieger R, Leyder M, Claus F, Leus A, De Catte L, Cossey V, Foulon I, Van der Valk E, Foulon W, Cos T, Bernaert A, Oyen R, Jani JC. 2016. Congenital cytomegalovirus infection: contribution and best timing of prenatal MR imaging. Eur Radiol 26:3760–3769. doi: 10.1007/s00330-015-4187-0. [DOI] [PubMed] [Google Scholar]