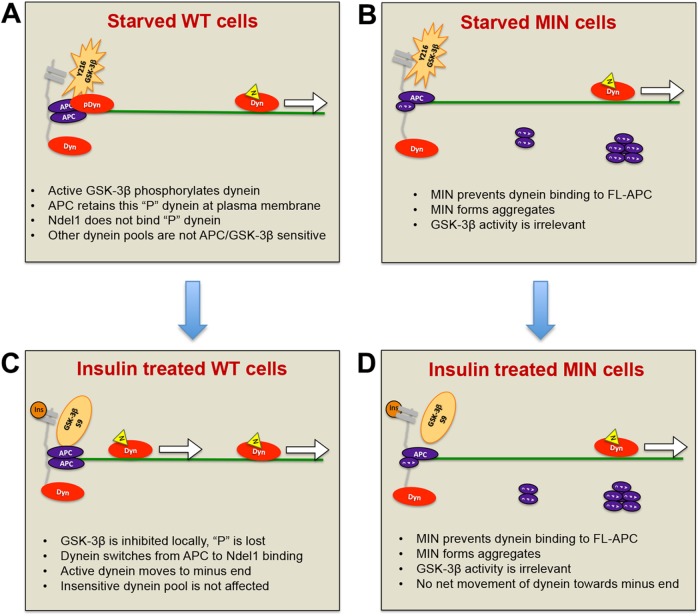
FIGURE 8:
Model for how APC mutations affect insulin-induced dynein movement toward MT minus ends. (A) WT cells in the absence of insulin or serum factors: GSK-3β (orange star) near the membrane (gray) is phosphorylated on Y216 and active. Nearby dynein motors are phosphorylated, rendering them more likely to bind to APC than to Ndel1. Here phosphorylated dynein (pDyn, red oval) is shown bound to an APC homodimer (purple ovals) at sites of MT (green line) capture at the plasma membrane. Unphosphorylated dynein (Dyn) may be bound to Ndel1 (yellow triangle) and actively translocating (arrow). (B) Insulin (dark orange circle) binds to receptors, activating a signaling pathway that locally and transiently inactivates GSK-3β by S9 phosphorylation. Dynein becomes locally and transiently dephosphorylated, is released from APC, binds to Ndel1, and then moves toward MT minus ends. The increase in number of processive dyneins results in minus-end accumulation at centrosomes (not shown). (C) MIN cells in the absence of insulin or serum factors: GSK-3β is active, but there is a reduced pool of dynein associated with FL-APC because MIN APC blocks the interaction. MIN APC also forms aggregates in the cytoplasm. (D) Normal inhibition of GSK3β occurs in response to insulin signaling but as the APC-bound, insulin-sensitive pool of dynein is reduced, the net effect of insulin on motor distribution is minimal.