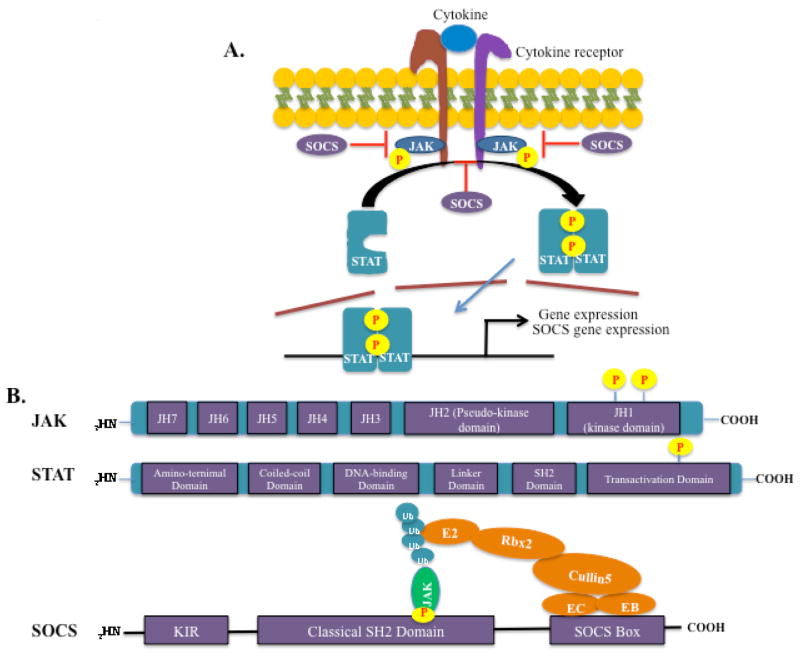
FIG. 1.
The JAK/STAT pathway and structure of JAK, STAT, and SOCS proteins. (A). Cytokine binding to its cognate receptor transactivates receptor-associated JAKs, lead to cross-phosphorylation and activation of the JAKs. The JAKs then phosphorylate the intracellular tail of the receptors on tyrosine residues, creating docking sites for the recruitment of STATs. STATs are then recruited to the phosphorylated receptor though the SH2 domain and tyrosine-phosphorylated by JAKs, resulting in STAT activation. Activated STATs will subsequently dimerize and translocate to the nucleus, where they bind to specific DNA elements and modulate the expression of target genes. SOCS proteins are not constitutively expressed; they are induced upon STAT activation and function in a negative feedback loop. (B). JAK, STAT, and SOCS protein domain structure. JAK proteins contain 7 JH domains including the pseudo-kinase domain (JH2) and the kinase domain (JH1). Trans- and autophosphorylation of tyrosine residues in the C-terminal kinase domain leads to the recruitment and activation of STATs. STAT proteins contain an aminoterminal domain, a coiled-coil domain, a DNA-binding domain, a linker domain, an SH2 domain, and a transactivation domain. Phosphorylation in the C-terminal transactivation domain by JAKs leads to STAT activation and dimerization. SOCS proteins contain a C-terminal conserved SOCS box, a classical SH2 domain, and an N-terminal variable length and organization region. The SOCS box interacts with components of the ubiquitin ligase machinery (Elongin B, Elongin C, Cullin-5, and Ring-box 2, and an E2 ubiquitin transferase), thereby mediating proteosomal degradation of associated target proteins, such as JAKs. SOCS1 and SOCS3 contain a kinase inhibitory region (KIR), which can directly inhibit JAK activity.