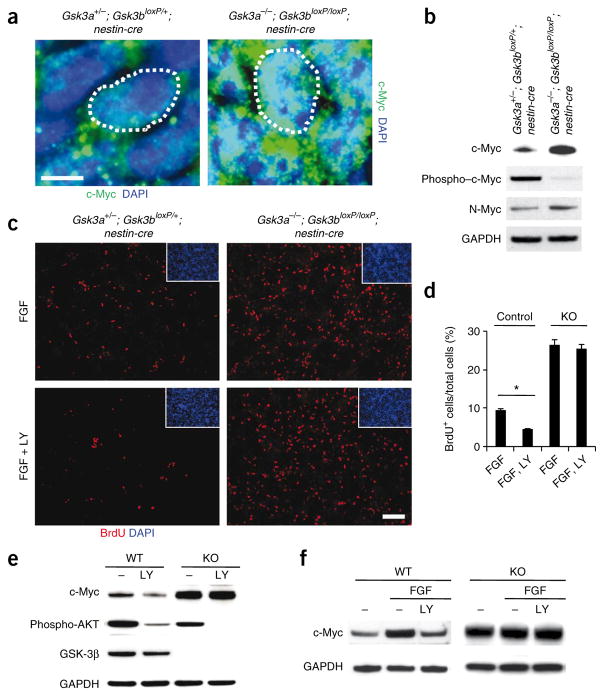
Figure 7.
GSK-3 mediates FGF-PI3K signaling to regulate c-Myc in neural progenitors. (a) Compared with control progenitors, c-Myc levels were massively increased in nuclei (white dotted circle) of Gsk3a−/−; Gsk3bloxP/loxP; nestin-cre progenitors at E14. Scale bar represents 5 μm. (b) Western blots showed that levels of c-Myc and N-Myc were increased in GSK-3 mutant brain lysates. In contrast, phospho–c-Myc was barely detectable in GSK-3 mutant brains. (c) FGF effects on progenitor proliferation were mediated in part by PI3K and GSK-3. Cortical progenitors from control and GSK-3 mutant tissues were cultured with either FGF2 alone or FGF2 and LY29400 (LY) for 24 h. Then, the cultures were treated with BrdU for 6 h and immunostained with an antibody to BrdU. Treatment with FGF increased progenitor proliferation in control cultures and PI3K inhibited the FGF2 effects. GSK-3 mutant progenitors showed no response to LY29400. Scale bar represents 70 μm. (d) The percentage of BrdU-positive cells was counted for quantification. * P < 0.01. (e) GSK-3 mediated PI3K regulation of c-Myc. Changes in c-Myc expression with LY294002 treatment were abrogated in Gsk3a−/−; Gsk3bloxP/loxP; nestin-cre cortical cultures. The decrease in phospho-AKT and phospho–GSK-3β showed the efficiency of PI3K inhibition. (f) GSK-3 mediated the effects of FGF signaling on c-Myc regulation. Western blots showed that the addition of a recombinant human basic FGF2 (10 ng ml−1) in control cultures markedly induced upregulation of c-Myc, which was suppressed by LY294002 treatment. However, neither the upregulation by FGF2 nor its suppression by LY294002 were observed in Gsk3a−/−; Gsk3bloxP/loxP; nestin-cre cortical cultures.