Abstract
Lymphedema is a chronic disease with a progressively ingravescent evolvement and an appearance of recurrent complications of acute lymphangitic type; in nature it is mostly erysipeloid and responsible for a further rapid increase in the volume and consistency of edema. The purpose of this work is to present our experience in the minimally invasive treatment for recurrence of lymphedema; adapting techniques performed in the past which included large fasciotomy with devastating results cosmetically; but these techniques have been proposed again by the use of endoscopic equipment borrowed from the advanced laparoscopy surgery, which allows a monoskin access of about one cm.
Keywords: Lymphedema, lymphatic venous anastomosis, transposition of the lymph nodes, microsurgery, supermicrosurgery, lymphoscintigraphy
1. Introduction
Lymphedema is a chronic disease with a progressively ingravescent evolvement and an appearance of recurrent complications of acute lymphangitic type;in nature it is mostly erysipeloid and responsible for a further rapid increase in the volume and consistency of edema [1].
The conservative therapeutic methods of medical and physical rehabilitation adopted in the treatment of lymphedema of the limbs allow to achieve some improvement in some types of lymphedema. In the recent decades, the literature shows that the advent of microsurgery and minimally invasive techniques, including autologous vascularized microsurgical lymph node (VLNT), the lymph lymphatic graft (Lympholymphatic graft), the lymphatic venous anastomosis (LVA) deep and the superficial performed in supermicrosurgery, have allowed positive and constantly prolonged results over time both in the treatment of primitive and secondary lymphedema [2].
Although the proposed techniques bring back consolidated and lasting results, according to various publications, and that 2-10% of patients treated surgically presents with recurrent lymphangitic episodes and in 15-20% of cases there is a recurrent disease, a variable percentage from 60-80% of cases have been able to discontinue the use of conservative measures [3].
The purpose of this work is to present our experience in the minimally invasive treatment for recurrence of lymphedema;adapting techniques performed in the past which included large fasciotomy with devastating results cosmetically, but these techniques have been proposed again by the use of endoscopic equipment borrowed from the advanced laparoscopy surgery, which allows a monoskin access of about one cm.
Since March 2014 we had operated this technique, and called it mini-invasive endospopic fasciotomy (MIF), on six patients with lymphedema of the lower limbs at II stage according to the classification of the International Society of Lymphology (ISL) subjected to previous surgery of LVA in another center with relapse after the first operation ranging from one to 15 years. All patients were underwent rehabilitation therapy before and after operation and still wear elastic compression.
The results obtained after ten months were improvements in term of volume post operating, and soft tissue of the affected limb.
2. Materials and methods
The patients we studied were suffering from chronic lymphedema of the unilateral lower limbs, previously subjected to a LVA intervention in another center and the disease recurred in a variable period after surgery from one to 15 years. Five patients were male and one was female with ages ranging from 22-67 years (mean age 44.8 years). All patients were subjected to medical, physical and rehabilitative treatment and the patients were classified as II stage according to the International Classification of the International Lymphology Society (ISL). Four patients were affected by primary lymphedema and two by secondary to surgery for cancer, one for prostate cancer and the second from bladder cancer (Table 1). Having already undergone a LVA surgery, it was decided to perform a wide fasciotomy minimally invasive leg. The technique is performed by endoscopic instrumentation, which is introduced through an incision of about one cm performed to 1/3 the average of the medial and lateral surface of the leg, below vision, and is then performed a wide fasciotomy of about 15 cm in length (Fig 1). One patient, also, has been subjected to plastic external genital for hydrocele of left testis and one to plastic penis with removal of edema tissue, and LVA anastomosis in bilateral inguinal supermi-crosurgey for reflux gravitational of external genitals with skin incision of about one cm, the lymphatic venous anastomosis side-to-side using nylon 10/0, it was performed under a microscope operator 20 X prior highlighting the lymphatic vessels with Blue pattent violet (Fig. 2). All patients have been subjected to biopsy of fascial tissue. The fasciotomy surgical intervention in minimally invasive is performed in spinal anesthesia, the total duration of it was about 35 minutes. All patients were discharged on the second post-operative day.
Table 1.
Clinical background of the patients
| Case | Age | Type of lymphedema | Stage | Time after LVA surgical operation | sex |
|---|---|---|---|---|---|
| 1 | 44 | Primary disease | II | 15 y | M |
| 2 | 63 | Bladder cancer | II | 6 y | M |
| 3 | 22 | Primary disease | II | 1 y | M |
| 4 | 50 | Primary disease | II | 7 y | F |
| 5 | 28 | Primary disease | II | 9 y | M |
| 6 | 62 | Prostate cancer | II | 2 y | M |
Figure 1.
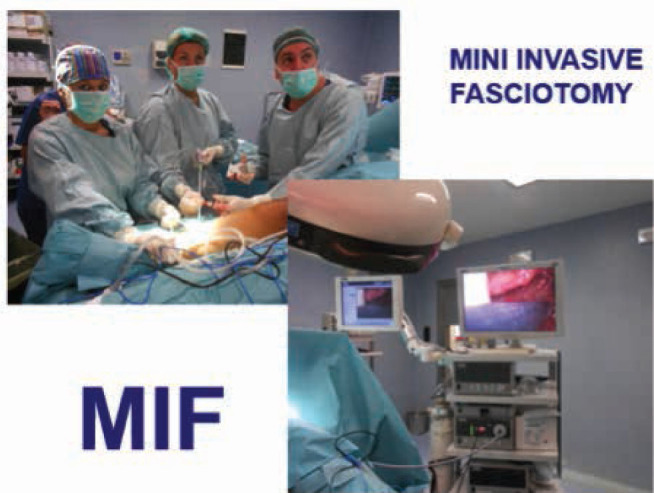
Mini invasive fasciotomy is performed by endoscopic technique, the skin incision of about 1 cm is performed at the middle third of the lateral and medial surface of the leg, the assistant holds camera and the first operator performs fasciotomy by mini laparoscopic scissors under vision.
Figure 2.
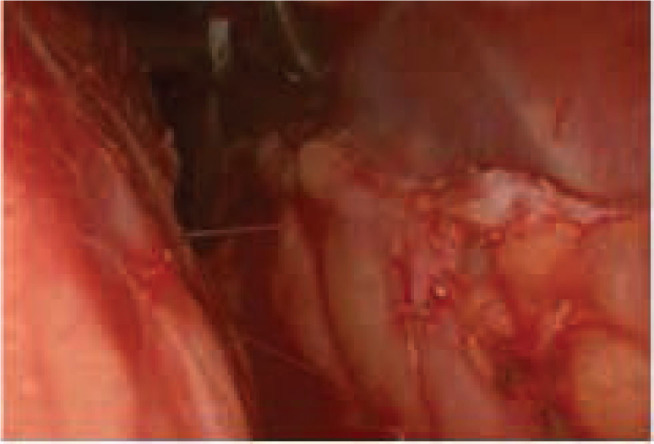
Fasciotomy endoscopic, is created a wide fenestration exposing under fascial plans
Ethical approval: The research related to human use has been complied with all the relevant national regulations, institutional policies and in accordance the tenets of the Helsinki Declaration, and has been approved by the authors’ institutional review board or equivalent committee.
Informed consent: Informed consent has been obtained from all individuals included in this study.
3. Results
The outcome of the patient, after MIF, was an immediate improvement in terms of reduction of edema and its consistency (Fig 3). All patients reported a reduction in the sensation of weight of the limb and there were no postoperative complications. Surgical scars have demonstrated a good cosmesi guaranteeing an excellent esthetic results. Histological examination showed sections of adipose-fibra tissue characterized by multifocal and marked expansion of the share collagenous-fibra, in the context of tissue you appreciate lymphatic vessels, a caliber of variable capillary formations to structures of increasing diameter, they often show a profile associated with irregular course abnormal wall thickening, due to hyperplasia of the tunica media, highlighted by strong immunoreactivity of the muscle cells for smooth muscle actin, which emphasizes the continuity and availablity in parallel bundles, to the load of the lymphatic structures described. It is also reported a slight well reinforcing component in elastic fibers (Fig. 4).
Figure 3.
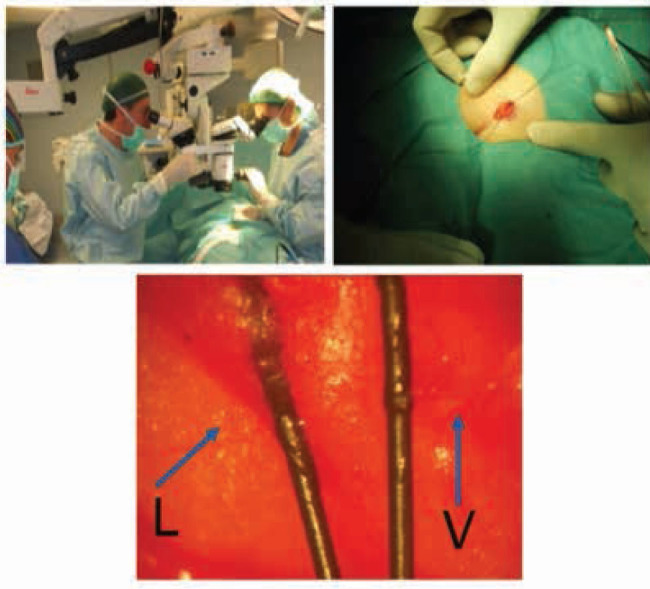
sequence for execution of lymphatic venous anastomosis by technique supermicrosurgery performed by mini incision of 1 cm and packed to the operating microscope 20 magnification, in detail L indicates the lymphatic and V the vein.
Figure 4.
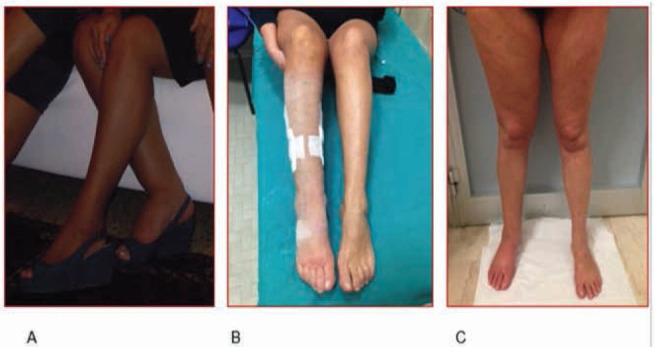
Follow-up at 10 months A) preoperative situation B) 10 days post operative c) 10 months post operative
Figure 5.
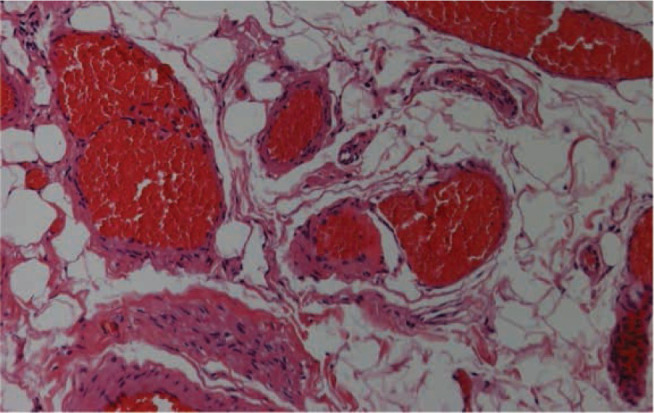
Histological examination: evidence of immunoreactivity of the muscle cells for actin and myosin dependent of lymphatic structures.
4. Discussion
Although the techniques of surgery for lymphedema have, for years now, consolidated with lasting results because the disease is chronic and progressing, the patients, over time, will have to undergo periodic specialist controls in any case who continues medical and rehabilitative therapy and fig-wear restraint elastic compression class variable depending on the clinical stage of patients. Despite the above results, a percentage ranging from 15 to 20% of patientsin the follow up have recurrent disease. The M.I.F. intervention aims to redress the unsatisfactory results of these patients and stands as additional modes surgical treatments of microsurgery [4,5].
The results, in the short and medium term, have been good with satisfaction of patients. This method has the advantage of being minimally invasive, which can be repeated if the need arises and the patient can resume after a week from the usual rehabilitation therapy by enhancing the beneficial effects obtained with surgery [6].
The ideal indications are the recidivists lymphedema at second stage, both primitive and secondary [7,8].
Our technique is performed in accordance with the lymph node structures that are preserved by not acting directly on them, but on the ability to exchange between the superficial and deep compartment that allows fasciotomy long fibrous tissues, enhancing the effect of the action of the pump muscle [9-11].
Clinical complications was not observed in any case. A shorter hospital stays means savings on days of hospitalization than the microsurgery that mean hospital stay of 4-5 days for the lymphatic-venous anastomoses performed in the groin crurale region for lymphedema of the lower limbs.
Conflict of interest statement
Authors state no conflict of interest
References
- [1].Becker C, Arrive L, Saaristo A, et al. Surgical treatment of congenital lymphedema. Clin Plast Surg. 2012 Oct;39(4):377–384. doi: 10.1016/j.cps.2012.08.001. [DOI] [PubMed] [Google Scholar]
- [2].Becker C, Assouad J, Riquet M, Hidden G. Postmastectomy lymphedema: long-term results following microsurgical lymph node transplantation. Ann Surg. 2006 Mar;243(3):313–315. doi: 10.1097/01.sla.0000201258.10304.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Boccardo F, Fulcheri E, Villa G. et al. Lymphatic microsurgery to treat lymphedema: techniques and indications for better results. Ann Plast Surg. 2013 Aug;71(2):191–195. doi: 10.1097/SAP.0b013e31824f20d4. [DOI] [PubMed] [Google Scholar]
- [4].Campisi C, Bellini C, Campisi C. et al. Microsurgery for lymphedema: clinical research and long-term results. Microsurgery. 2010 May;30(4):256–260. doi: 10.1002/micr.20737. [DOI] [PubMed] [Google Scholar]
- [5].Campisi C, Eretta C, Pertile D. et al. Microsurgery for treatment of peripheral lymphedema: long-term outcome and future perspectives. Microsurgery. 2007;27(4):333–338. doi: 10.1002/micr.20346. [DOI] [PubMed] [Google Scholar]
- [6].Földi E. Terapy of lymphedema. Hautarzt. 2012 Aug;63(8):627–633. doi: 10.1007/s00105-012-2364-5. [DOI] [PubMed] [Google Scholar]
- [7].Mihara M, Hara H, Kikuchi K. et al. Scarless lymphatic venous anastomosis for latent and early-stage lymphoedema using indocyanine green lymphography and non-invasive instruments for visualising subcutaneous vein. J Plast Reconstr Aesthet Surg. 2012 Nov;65(11):1551–1558. doi: 10.1016/j.bjps.2012.05.026. [DOI] [PubMed] [Google Scholar]
- [8].Mihara M, Murai N, Hayashi Y. et al. Using indocyanine green fluorescent lymphography and lymphatic-venous anastomosis for cancer-related lymphedema. Ann Vasc Surg. 2012;26(2):278. doi: 10.1016/j.avsg.2011.08.007. [DOI] [PubMed] [Google Scholar]
- [9].Thompson N. The surgical treatment of chronic lymphoedema of the extremities. Surg Clin North Am. 1967 Apr;47(2):445–503. doi: 10.1016/s0039-6109(16)38188-9. [DOI] [PubMed] [Google Scholar]
- [10].Serra R, Buffone G, Perri P. et al. Male breast cancer manifesting as cephalic vein thrombosis. Annals of Vascular Surgery. 2013;27(8):1188.e9–1188.e11. doi: 10.1016/j.avsg.2013.01.016. [DOI] [PubMed] [Google Scholar]
- [11].Rocco N, Della Corte GA, Rispoli C. et al. Axillary masses in a woman with a history of breast cancer: dermatopathic lymphadenopathy. Int J Surg. 2014;12:S40–43. doi: 10.1016/j.ijsu.2014.08.384. Suppl 2. [DOI] [PubMed] [Google Scholar]


