Abstract
Objective
The aim of this study was to investigate the correlation of von Hippel-Lindau tumor suppressor (VHL) mRNA expression and jade family PHD finger 1 (Jade-1) gene expression in patients with renal cell carcinoma (RCC). Another aim of this study was to analyze the relationship of these two genes with clinicalpathological features of the RCC patients.
Methods
A total of 75 RCC patients who received surgically therapy in our hospital were included. All patients had complete pathological data. The expression of VHL/Jade-1 was determined by real-time polymerase chain reaction (RT-PCR).
Results
VHL and Jade-1 were both obviously downregulated in RCC tissues than that of the matched normal tissues, and both negatively correlated with tumor size as well as tumor grade. And we found a fine association of VHL gene expression with Jade-1.
Conclusion
VHL/Jade-1 exhibited significantly decreased expression in RCC tissues and was closely related to the clinical prognosis of patients. The finding of VHL expression positively correlated with Jade-1 expression shed light and provided crucial evidence on the connection of VHL protein with Wnt/b-catenin pathway.
Keywords: VHL, Jade-1, RCC, correlation, Wnt/b-catenin
1. Introduction
Renal cell carcinoma (RCC) ranks as the most frequent type of all kidney cancers, which represents more than 85% incidence among kidney cancers [1,2]. It is also the ninth most common cancer in developed countries [3]. In recent years, the incidence of RCC shows a rising trend. Recent progress in antiangiogenic therapy has increased the median survival of RCC patients at advance stages from 10 months to more than 40 months [4]. However, because of the highly expression of drug-resistant genes, general chemotherapy method has limited or absolutely no significant effect [5,6]. Therefore, deeper understanding of the pathogenesis and progression of RCC is of highly importance for developing more therapy targets and improving prognosis of RCC patients.
Von Hippel–Lindau (VHL) disease is a hereditary cancer syndrome caused by inherited mutations that inactivate the VHL tumour suppressor gene [7]. Patients with VHL disease are with relative higher risk for a variety of cancers, including RCC of the clear cell histology and other kinds of cancers [8]. The best-characterized function of VHL refers to targeting of the hypoxia-inducible factor (HIF) transcription factor for proteolytic degradation [9,10]. However, VHL inactivation mediates both HIF–dependent and –independent pathways [11-14]. Notably, the majority of the HIF-independent functions were discovered through biochemical interactions, among which, VHL had been reported to interact with and stabilize Jade-1.
Jade-1, a short-lived protein most highly expressed in renal proximal tubules, is a candidate renal tumor sup-pressor. It was initially identified by yeast two-hybrid analysis as a VHL-interacting protein [15]. Jade-1 could suppress renal cancer cell growth in part by increasing apoptosis [16]. It functions as: 1) a ubiquitin ligase to inhibit canonical Wnt/b-catenin signaling [17]; 2) a transcription factor associated with histone acetyltransferase activity [18]; 3) with increased abundance of cyclin-dependent kinase inhibitor p21 [19]. The relationship between Jade-1 and VHL has not yet to be elucidated fully, and may have important implications for RCC development [16]. Recent findings of ubiquitylation followed by degradation of b-catenin by Jade-1 provided evidence that loss of VHL could lead to tumor formation through b-catenin de-repression [20].
However, the connection of VHL expression with Jade-1 expression, and their clinical significance in RCC remain unclear. Here we sought to give direct evidence to demonstrate their correlations and characterize their clinical value in RCC patients.
2. Materials and methods
2.1. Patients and tissues
A total of 75 RCC tissues, as well as adjacent normal tissues from patients with RCC pathologically confirmed were collected from 2012 to 2015. Characteristic data for sex, age, tumor size, lymph node (LN) metastasis and tumor grade of patients were obtained from patients’ medical records. The study protocol was complied with the ethical guidelines of The Central Hospital of Lishui City.
Informed consent: Informed consent has been obtained from all individuals included in this study.
2.2. RNA extraction, Reverse transcription and Real-Time PCR
Total RNA of tissues was extracted with TRIzol reagent (Invitrogen, Carlsbad, CA). The quantitation and quality of RNA was determined using Nanodrop 2000c spectrophotometer. Reverse transcription of RNA was done. Real-Time PCR analysis was performed with SYBR Green qPCR mix (TOYOBO). Samples were normalized to β-actin. Relative VHL or Jade-1 expression was calculated using the power formula: 2-∆Ct (∆Ct = CtGene – Ctβ-actin).
2.3. Statistical analysis
All statistical analyses were performed using SAS software v8. The significance of differences between cancer and normal tissues was estimated by Paired Student’s t-test. κ 2 test was performed to test the relationship between gene expression and clinical parameters. Correlation between expression levels of VHL and Jade-1 genes in RCC tissues was analyzed using Spearman’s linear correlation. Two-sided P-values were calculated, and a probability level of 0.05 was chosen for statistical significance.
3. Results
3.1. VHL and Jade-1 expressions were decreased in RCC and negatively correlated with advanced clinical stage
Real-time PCR was performed to detect the expression of VHL and Jade-1 in 75 pairs of human RCC tissues. We found that 62.7% (47/75) of VHL expression was downregulated in RCC tissues when compared with adjacent normal tissues (Figure 1A, t-test, P<0.01). Also, 60% (45/75) of Jade-1 expression in RCC tissues was significantly down-regulated (Figure 1B, t-test, P<0.01).
Figure 1.
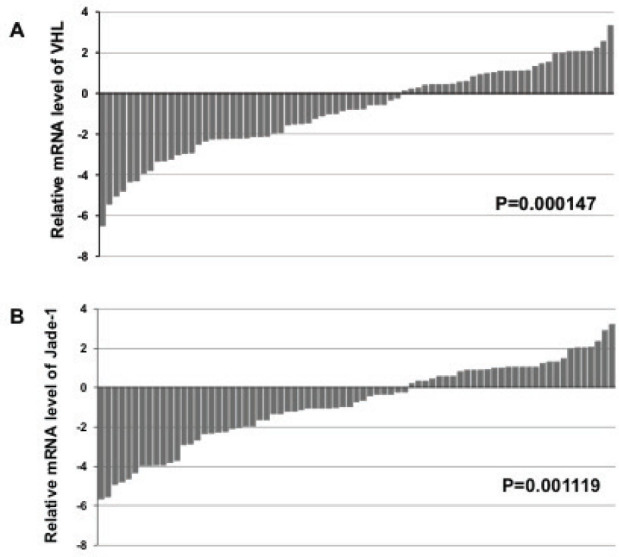
The status and expression levels of VHL and Jade-1 in RCC and adjacent normal tissues. Comparison of relative VHL (A)and Jade-1 (B)expression levels as assessed by real-Time PCR between 75 RCCs and adjacent normal tissues. The relative mRNA expression levels in kidney tumors are presented as fold change = 2∆CtN-∆CtT of tumors versus matched normal tissues.
Then, patients with RCC were classified into two groups based on the DDCt of relative VHL expression or Jade-1 expression (positive or negative expression). As showed in Table 1, VHL expression was significantly associated with tumor size and tumor grade (Table 1, k2 test, P<0.01), but no significant association with patients’ sex, age or LN metastasis (Table 1, k2 test, P>0.05). However, Jade-1 expression was not only significantly associated with tumor size and tumor grade (Table 1, k2 test, P<0.01), but also associated with LN metastasis (Table 1, k2 test, P<0.01). Taken together, these results indicated that VHL and Jade-1 were downregulated in RCC, and reduced expression of them may play tumor suppressive roles in the progression of RCC.
Table 1.
Relationship between mRNA expression of both VHL and Jade-1, and age, sex, tumor size, lymph node metastasis and tumor grade in RCC.
| Variables | Total number of cases | VHL positive n (%) | VHL negative n (%) | P | Jade-1 positive n(%) | Jade-1 negative n(%) | P |
|---|---|---|---|---|---|---|---|
| 28 (37.3%) | 47 (62.7%) | 30 (40.0%) | 45 (60.0%) | ||||
| Age | |||||||
| <=50 | 29 | 13 | 16 | 12 | 17 | ||
| >50 | 46 | 15 | 31 | 0.3324 | 18 | 28 | 1.0000 |
| Sex | |||||||
| Male | 44 | 16 | 28 | 19 | 25 | ||
| Female | 31 | 12 | 19 | 1.0000 | 11 | 20 | 0.6331 |
| Tumor Size | |||||||
| <=4cm | 33 | 19 | 14 | 19 | 14 | ||
| >4cm | 42 | 9 | 33 | 0.0018 | 11 | 31 | 0.0088 |
| LN metastasis | |||||||
| Yes | 35 | 15 | 20 | 8 | 27 | ||
| No | 40 | 13 | 27 | 0.4734 | 22 | 18 | 0.0053 |
| Tumor Grade | |||||||
| I | 15 | 12 | 3 | 13 | 2 | ||
| II | 25 | 8 | 17 | 9 | 16 | ||
| III+IV | 35 | 8 | 27 | 0.0005 | 8 | 27 | 0.0001 |
3.2. VHL was positively correlated with Jade-1 expression in RCC
To further explore the relationship between VHL and Jade-1, Spearman’s correlation analysis was performed by SAS software. The results indicated that there was a positively correlation between VHL and Jade-1 expression (Figure 2, P<0.0001). Our data demonstrated that Jade-1 might be positively regulated by VHL, or they might be involved in one pathway.
Figure 2.
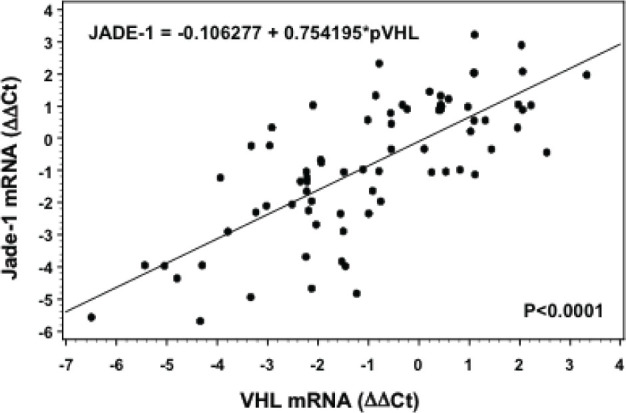
The positive correlation between VHL and Jade-1 expression in 75 RCC samples was determined using Spearman’s correlation analysis (P<0.0001).
4. Discussion
The familial cancer syndrome, VHL disease, occurs as a consequence of inheriting a mutation in the VHL tumour-suppressor gene located on chromosome locus 3p257. VHL is frequently inactivated in sporadic cases of clear-cell RCC. Its genetic alterations, including gene mutations, hypermethylation of the promoter region, and loss of heterozygosity at the VHL locus are common genetic events in sporadic cases of clear-cell RCC. Clear-cell RCC is the major subtype of RCC. The findings in clear-cell RCC might be applied into the total satiation of RCC.
In our study, we determined the mRNA expression of VHL as well as its partner Jade-1 in RCC tissues, matched with paired normal tissues as controls. We found that both of them exhibited decreased expression in RCC tissues. And the expression level of VHL positively correlated with that of Jade-1, indicating a positive regulation of Jade-1 by VHL in RCC cells. This phenomenon was ideally and in line with our expectation, since it was already known that VHL could interact with and stabilize Jade-1 [15]. As the upstream regulator, VHL to some extent would decide at least protein levels of Jade-1. However, why VHL could also regulate the mRNA expression of Jade-1 remains unclear. Some other mechanisms should be involved in this process. Nevertheless, we do believe that, VHL might function as a tumor suppressor in RCC through Jade-1 to regulate the Wnt/b-catenin signaling. Actually, only one previous report has discussed the interplay between VHL/ HIF-1alpha and Wnt/b-catenin pathways during colorectal tumorigenesis [21]. Therefore, our results provide another possibility to interpret the crosstalk of VHL with Wnt/b-catenin pathway in RCC.
We also analyzed the relationships of VHL and Jade-1 with clinical parameters of the collected RCC patients. We surprisingly found that both VHL and Jade-1 significantly correlated with tumor size and tumor grade, which further confirmed their tumor suppressor roles in RCC. Additionally, we found that only Jade-1 was correlated with lymph node metastasis parameters, indicating that besides promoting apoptosis, Jade-1 might also participate in metastasis processes in RCC cells.
The limited aspect of our study is that, we merely detect the mRNA expression of the two molecules. Indeed, it is more important to address the gene mutation, DNA methylation or protein expression of VHL in RCC tissues. Considering the huge workload, we just measured the mRNA levels of VHL. Hence, for the follow-up studies, we will focus more on genetic and epigenetic levels of VHL regulation. It is the same for Jade-1 studies.
In summary, our results reported the correlation of VHL with Jade-1 in RCC tissues, both of which were decreased, and accessed their clinical values by analyzing the relationship between mRNA expression of VHL and/or Jade-1 and clinical parameters of RCC patients. Our findings provide another clue to connect VHL and Wnt/b-catenin pathway in RCC.
Conflict of interest statement
Authors state no conflict of interest
References
- [1].De P, Otterstatter MC, Semenciw R, Ellison LF, Marrett LD, Dryer D. Trends in incidence, mortality, and survival for kidney cancer in Canada, 1986-2007. Cancer Causes Control. 2014 Oct;25(10):1271–1281. doi: 10.1007/s10552-014-0427-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Davis ID. Advanced renal cell carcinoma in Australia, 2015: what should we. Asia Pac J Clin Oncol. 2014 Dec;10(4):285–288. doi: 10.1111/ajco.12314. [DOI] [PubMed] [Google Scholar]
- [3].GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015 Jan;10-385(9963):117–171. doi: 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Thomas JS, Kabbinavar F. Metastatic clear cell renal cell carcinoma: A review of current therapies and novel immunotherapies. Crit Rev Oncol Hematol. 2015 Jul;20:S1040–8428(15)30009-3. doi: 10.1016/j.critrevonc.2015.07.009. [DOI] [PubMed] [Google Scholar]
- [5].Diamond E, Molina AM, Carbonaro M, Akhtar NH, Giannakakou P, Tagawa ST, Nanus DM. Cytotoxic chemotherapy in the treatment of advanced renal cell carcinoma in the era of targeted therapy. Crit Rev Oncol Hematol. 2015 Aug;13:S1040–8428(15)30022-6. doi: 10.1016/j.critrevonc.2015.08.007. [DOI] [PubMed] [Google Scholar]
- [6].van der Mijn JC, Mier JW, Broxterman HJ, Verheul HM. Predictive biomarkers in renal cell cancer: insights in drug resistance mechanisms. Drug Resist Updat. 2014 Oct-Dec;17(4-6):77–88. doi: 10.1016/j.drup.2014.10.003. [DOI] [PubMed] [Google Scholar]
- [7].Latif F, Tory K, Gnarra J, Yao M, Duh FM, Orcutt ML, Stackhouse T, Kuzmin I, Modi W, Geil L. et al. Identification of the von Hippel-Lindau disease tumor suppressor gene. Science. 1993 May 28;260(5112):1317–1320. doi: 10.1126/science.8493574. [DOI] [PubMed] [Google Scholar]
- [8].Kim WY, Kaelin WG. Role of VHL gene mutation in human cancer. J Clin Oncol. 2004 Dec 15;22(24):4991–5004. doi: 10.1200/JCO.2004.05.061. [DOI] [PubMed] [Google Scholar]
- [9].Li M, Kim WY. Two sides to every story: the HIF-dependent and HIF-independent functions of pVHL. J Cell Mol Med. 2011 Feb;15(2):187–195. doi: 10.1111/j.1582-4934.2010.01238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Haase VH. The VHL tumor suppressor: master regulator of HIF. Curr Pharm Des. 2009;15(33):3895–903. doi: 10.2174/138161209789649394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME, Wykoff CC, Pugh CW, Maher ER, Ratcliffe PJ. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999 May 20;399(6733):271–275. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- [12].Gordan JD, Lal P, Dondeti VR, Letrero R, Parekh KN, Oquendo CE, Greenberg RA, Flaherty KT, Rathmell WK, Keith B, Simon MC, Nathanson KL. HIF-alpha effects on c-Myc distinguish two subtypes of sporadic VHL-deficient clear cell renal carcinoma. Cancer Cell. 2008 Dec 9;14(6):435–446. doi: 10.1016/j.ccr.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Jiang Y, Zhang W, Kondo K, Klco JM, St Martin TB, Dufault MR, Madden SL, Kaelin WG Jr, Nacht M. Gene expression profiling in a renal cell carcinoma cell line: dissecting VHL and hypoxia-dependent pathways. Mol Cancer Res. 2003 Apr;1(6):453–462. [PubMed] [Google Scholar]
- [14].Zatyka M, da Silva NF, Clifford SC, Morris MR, Wiesener MS, Eckardt KU, Houlston RS, Richards FM, Latif F, Maher ER. Identification of cyclin D1 and other novel targets for the von Hippel-Lindau tumor suppressor gene by expression array analysis and investigation of cyclin D1 genotype as a modifier in von Hippel-Lindau disease. Cancer Res. 2002 Jul 1;62(13):3803–3811. [PubMed] [Google Scholar]
- [15].Zhou MI, Wang H, Ross JJ, Kuzmin I, Xu C, Cohen HT. The von Hippel-Lindau tumor suppressor stabilizes novel plant homeodomain protein Jade-1. J Biol Chem. 2002 Oct 18;277(42):39887–39898. doi: 10.1074/jbc.M205040200. [DOI] [PubMed] [Google Scholar]
- [16].Zhou MI, Foy RL, Chitalia VC, Zhao J, Panchenko MV, Wang H, Cohen HT. Jade-1, a candidate renal tumor suppressor that promotes apoptosis. Proc Natl Acad Sci USA. 2005 Aug 2;102(31):11035–11040. doi: 10.1073/pnas.0500757102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Chitalia VC, Foy RL, Bachschmid MM, Zeng L, Panchenko MV, Zhou MI, Bharti A, Seldin DC, Lecker SH, Dominguez I, Cohen HT. Jade-1 inhibits Wnt signalling by ubiquitylating beta-catenin and mediates Wnt pathway inhibition by pVHL. Nat Cell Biol. 2008 Oct;10(10):1208–1216. doi: 10.1038/ncb1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Panchenko MV, Zhou MI, Cohen HT. von Hippel-Lindau partner Jade-1 is a transcriptional co-activator associated with histone acetyltransferase activity. J Biol Chem. 2004 Dec 31;279(53):56032–56041. doi: 10.1074/jbc.M410487200. [DOI] [PubMed] [Google Scholar]
- [19].Foy RL, Chitalia VC, Panchenko MV, Zeng L, Lopez D, Lee JW, Rana SV, Boletta A, Qian F, Tsiokas L, Piontek KB, Germino GG, Zhou MI, Cohen HT. Polycystin-1 regulates the stability and ubiquitination of transcription factor Jade-1. Hum Mol Genet. 2012 Dec 15;21(26):5456–5471. doi: 10.1093/hmg/dds391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Berndt JD, Moon RT, Major MB. Beta-catenin gets jaded and von Hippel-Lindau is to blame. Trends Biochem Sci. 2009 Mar;34(3):101–104. doi: 10.1016/j.tibs.2008.12.002. [DOI] [PubMed] [Google Scholar]
- [21].Giles RH, Lolkema MP, Snijckers CM, Belderbos M, van der Groep P, Mans DA, van Beest M, van Noort M, Goldschmeding R, van Diest PJ, Clevers H, Voest EE. Interplay between VHL/ HIF1alpha and Wnt/beta-catenin pathways during colorectal tumorigenesis. Oncogene. 2006 May;18-25(21):3065–3070. doi: 10.1038/sj.onc.1209330. [DOI] [PubMed] [Google Scholar]


