Abstract
Aim:
The present study was carried out to examine this hypothesis that administration of selenium can prevent the development of injuries by brain trauma and thus can modulate patients’ functional recovery and also improve posttraumatic outcome.
Materials and Methods:
This double-blinded controlled trial was carried out on 113 patients who were hospitalized following traumatic brain injury (TBI) with Glasgow Coma Scale score of 4–12 that were randomly assigned to receive selenium within 8 h after injury plus standard treatment group or routine standard treatment alone as the control. The primary endpoint was to assess patients’ functional recovery at 2 months after the injury based on extended Glasgow Outcome Scale score (GOS-E). Secondary outcomes included the changes in Full Outline of Unresponsiveness score (FOUR) score, Sequential Organ Failure Assessment (SOFA) score, and acute physiology and chronic health evaluation (APACHE) III score, side effects of selenium, length of Intensive Care Unit (ICU) stay, and length of hospital stay.
Results:
There was no difference in the length of ICU and hospital stay, the trend of the change in FOUR and SOFA scores within 15 days of first interventions, and the mean APACHE III score on the 1st and 15th days between the two groups. Mortality was 15.8% in selenium group and 19.6% in control group with no between-group difference. No difference was revealed between the two groups in appropriate outcome according to GOS-E score at 60 ± 10 days and also 30 ± 5 days according to the severity of TBI.
Conclusion:
This human trial study could not demonstrate beneficial effects of intravenous infusion of selenium in the improvement of outcomes in patients with acute TBI.
Keywords: Acute traumatic brain injury, extended glasgow outcome scale, selenium
Introduction
Traumatic brain injury (TBI) accounts for more than one million admissions to emergency centers leading to more than 50,000 deaths as well as millions disabilities annually in the world.[1] Both genders and different age subgroups can be affected by this problem with different mechanisms. Due to the necessity for proper management of these injuries, understanding pathophysiology, mechanisms, and also recent diagnostic and therapeutic modalities is certainly critical. Pathophysiologically, TBIs can be classified as the primary with direct damaging the cerebrovascular tissues leading to direct neuronal injuries or the secondary to cerebral responses to other baseline pathological conditions such as cerebral edema, infections, hemorrhages, cerebral hypoxia, or increased intracranial pressure.[2,3,4] Eventually, both types of injuries may result in ischemic or hemorrhagic cerebral events or neuronal death. Along with pathophysiological changes following brain injury, some changes have been also identified in molecular basis.[5] Both primary and secondary mechanisms of injuries commonly generate reactive oxygen species (ROS) that mediate oxidative stress leading to neural dysfunction and even neuronal death.[6] In this regard, the excessive expression and production of ROS following exhaustion of the endogenous antioxidant system can induce cellular and vascular peroxidation, DNA cleavage, and inhibition of the mitochondrial electron transport chain in background of brain injuries.[7,8] In other words, because balanced production of ROS is essential for brain enzymes’ activities and cell signaling, the imbalance between oxidants and antioxidants and thus excessive production of ROS may be disrupted following injuries. Thus, defense mechanisms based on controlling ROS production and creating balance between oxidants and antioxidants are the main aim for controlling the cellular consequences resulting from brain trauma injuries.[9] The critical role of selenium in creating this balance has been exclusively focused. It has been revealed that selenium dependent to glutathione (GSH) has a central antioxidant role that prevents cellular damages caused by ROS excessive production.[10,11] In fact, GSH activity can modulate body selenium level by balancing ROS that is necessary for preventing development of neural defects following brain injuries. Based on these evidences, it is now suggested that intake of selenium supplements may bolster preventive mechanisms against cellular injuries caused by misbalancing between oxidants and antioxidants.[12] Accordingly, the present study was carried out to examine this hypothesis that administration of selenium can prevent the development of injuries by brain trauma and thus can modulate patients’ functional recovery and also improve posttraumatic outcome.
Materials and Methods
Study population
After obtaining approval from the Ethics Committee of Iran University of Medical Sciences (IR. IUMS.rec. 1394.9311692002) and also getting written informed consent in accordance with national legislation (from the alert patients or from the patient's legal representative for unconscious condition), this randomized double-blinded controlled trial was performed with IRCT registration number of IRCT2015090223865N1. The study population included the patients aged higher than 18 years that were hospitalized following brain trauma with Glasgow Coma Scale (GCS) score 12 or less and with at least one pupil reaction to light. The exclusion criteria were GCS of 3, no bilateral pupil reaction to light, chance of live being of <24 h, the presence of hypotension (systolic blood pressure <90 mmHg) for more than 10 min, spinal cord injury, pregnancy, single epidural hematoma, or history of renal dysfunction.
Intervention protocol
The participants were randomly (through simple randomization, according to the first right digit of national code that could be even or odd) assigned into two groups as the case group (that received selenium [Selenase, Biosyn co., Germany] 500 µg intravenously at 100 ml normal saline for 30 min and then 500 µg at 100 ml normal saline during 24 h continuously for 14 days in addition to standard care) and the control group (that benefited only from standard care). The standard treatment in both groups consisted of respiratory support, prevention of seizure, fluid and electrolyte balancing, and prevention of stress ulcers and deep vein thrombosis. If there was evidence of cerebral edema in computed tomography (CT) scan, proper treatment (head elevation, osmotic therapy, diuretic therapy, hyperventilation, analgesics, sedatives, and cerebrospinal fluid drainage) was considered. Both infused solutions had similar color and volume (100 ml) and were marked with code A or B.
Study measurements and endpoints
For all participants, acute physiology and chronic health evaluation (APACHE) III score was determined on the 1st and 15th days. Since the beginning of the study to 15 days, the level of consciousness was daily determined based on FOUR score, and functional status of the organs was also determined on Sequential Organ Failure Assessment (SOFA) score. The patients were also assessed regarding selenium side effects including nausea, vomiting, nail changes, hair loss, the smell of garlic, and facial flushing. The primary endpoint was to assess patients’ functional recovery in 60 ± 10 days after TBI based on extended Glasgow Outcome Scale (GOS-E) scale. In this scaling system, the recovery was leveled as E1 (death), E2 (vegetative status), E3 and E4 (severe disability), E5 and E6 (moderate disability), and E7 and E8 (good recovery). Appropriate recovery was defined as GOS-E ≥7 in patients with moderate injuries (GCS of 9–12), GOS-E ≥5 in patients with moderate to severe injuries (GCS of 6–8), and GOS-E ≥3 in patients with severe injuries (GCS of 4 or 5). The second endpoint was to determine and compare the change in APACHE III score on the 15th day compared to the 1st day, the daily changes in FOUR score and SOFA score within 15 days of first interventions, side effects of selenium, length of Intensive Care Unit (ICU) stay, and length of hospital stay between the two groups.
Statistical analysis
For statistical analysis, results were presented as mean ± standard deviation for quantitative variables and were summarized by absolute frequencies and percentages for categorical variables. Normality of data was analyzed using Kolmogorov–Smirnov test. Categorical variables were compared using Chi-square test or Fisher's exact test when more than 20% of cells with expected count of <5 were observed. Quantitative variables were also compared with t-test or Mann–Whitney U-test. For the statistical analysis, the statistical software SPSS version 16.0 for windows (SPSS Inc., Chicago, IL, USA) was used. P ≤ 0.05 were considered statistically significant.
Results
In total, 113 patients were randomized to two selenium (n = 57) and control (n = 56) groups [Table 1]. The two groups were similar in male gender (78.9% vs. 80.4%, P = 0.85) and mean age (40.07 ± 17.82 vs. 42.93 ± 17.19 years, P = 0.38). The most frequent cause of brain trauma in selenium and control groups included traffic accident (71.9% vs. 71.4%), followed by falling (21.1% vs. 17.9%), dispute (3.5% vs. 5.4%), and other causes (3.5% vs. 5.4%) without between-group difference (P = 0.90). The frequencies of single brain and multiple trauma were 77% and 23% in selenium group and 75% and 23% in control group with no difference (P = 0.66). There was no difference in the mean GCS score at baseline between selenium group (8.00 ± 2.23) and control group (8.32 ± 2.31) (P = 0.68). Regarding severity of injury in case and control groups, severe injury was found in 15.8% and 12.5%, moderate-to-severe injury in 43.9% and 39.3%, and moderate injury in 40.4% and 48.2%, respectively (P = 0.68). There was also no difference in mean size of pupils at left- and right-sided as well as reaction to light [Table 1]. The frequency of brain edema in selenium group was 35.1% and in control group was 28.6% with no difference (P = 0.45). There was also no difference between the groups in Marshall scoring at the first CT scan, craniotomy, external ventricular drainage, and mean blood pressure. The mean time for mechanical ventilation was 8.44 ± 8.09 days in selenium group and 9.30 ± 11.20 days in another group (P = 0.63). There was also no difference in mean length of ICU stay (14.51 ± 8.01 vs. 15.91 ± 13.24 days, P = 0.49) as well as in mean length of hospital stay (19.40 ± 8.76 vs. 20.04 ± 13.14 days, P = 0.76) between the group received selenium and the group received routine treatment alone. As shown in Figure 1, the mean FOUR score gradually increased from the 1st day to the 15th day without difference in the trend of the changes between the selenium and control groups. Adversely, the mean SOFA score gradually decreased within 15 days of first interventions without any between-group difference [Figure 2]. The mean APACHE III score on the 1st and 15th days in selenium group was 49.91 ± 17.12 and 30.55 ± 17.32 and in control group was 49.34 ± 18.13 and 25.50 ± 15.60 with no difference between groups (P = 0.86 and P = 0.16), respectively. With respect to drug-induced side effects, nausea was found in one patient and facial flushing in three patients who receiving selenium. In total, 15.8% in selenium group and 19.6% in control group died (P = 0.59). The mortality rate in selenium and control group was 66.7% and 42.9% in severe injuries (P = 0.34), 0.0% and 13.6% in moderate-to-severe injuries (P = 0.56), and 13.0% and 18.5% in moderate injuries (P = 59), respectively. As indicated in Table 2, no difference was revealed between the two interventional groups in appropriate outcome according to GOS-E score at 60 ± 10 days and also 30 ± 5 days according to the severity of traumatic injury. In this regard, severe disability was revealed 42.1% and 41.1% and good recovery in 24.6% and 19.6% of patients in selenium and control group, respectively, with no significant difference.
Table 1.
Baseline information in selenium and control groups
Figure 1.
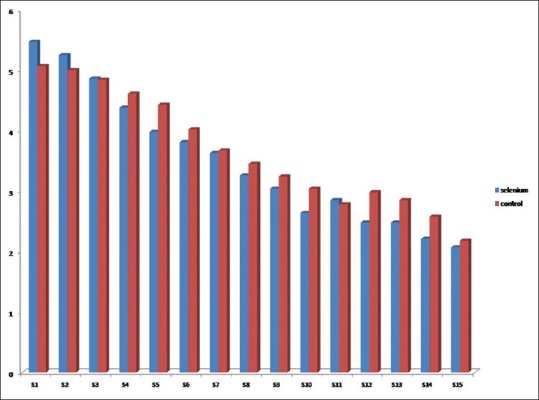
Trend of the changes in FOUR score in selenium and control groups.
Figure 2.
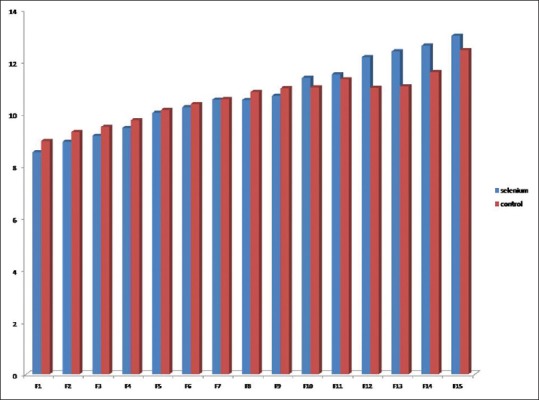
Trend of the changes in Sequential Organ Failure Assessment score in selenium and control groups.
Table 2.
Baseline information in selenium and control groups
Discussion
Because of beneficial effects of selenium use in balancing oxidants and antioxidants and thus its impact on regulating ROS function, we hypothesized that administrating selenium as intravenous infusion might improve outcome in patients who were hospitalized due to TBIs. In other words, along with standard supportive protocols and treatment approaches in these patients, using intravenous selenium on admission may lead to lower mortality and morbidity. In the present study, administration of 500 µg selenium per day for 14 days in addition to standard treatment could not change patients’ outcome including functional recovery, mean time for mechanical ventilation, level of consciousness, and also living status and length of hospital and ICU stay. As previously pointed, TBI as a main reason for mortality and disability in the world has been identified as a progressive disorder with primary defects such as intracranial hemorrhage, contusion, or diffuse cerebral defects after trauma led to secondary injuries. The basis of metabolic disturbances includes the production of free oxygen radicals, stimulatory amino acids (aspartate and glutamate), inflammatory cytokines (interleukins 1 and 6 [IL 1 and 6] and tumor necrosis factor-alpha), and finally brain edema and tissue damages.
Despite employing different treatment protocols for improving outcome of traumatic injuries, most clinical trials with the purpose of inhibiting secondary factors such as lipid peroxidation (steroids and tirilazad), free radicals (superoxide dismutase), calcium channel blocking (nimodipine), glutamate (selfotel), apoptosis (cyclosporine A), and edema (substance P antagonists) had limited success.[8] The present study is the first human clinical trial that assessed the therapeutic effect of selenium. Previous studies were carried out as animal studies that could show significant effects of selenium as brain tissue support. In a recent study by Naziroglu et al.,[10] it was shown that selenium as a thiol redox system antioxidant can protect brain tissue against inflammation and stress oxidative. They could show that the effect of selenium can be induced by affecting apoptosis, oxidative stress, and Ca (2+) influx through TRPV1 channel activations in brain tissue, particularly in the hippocampus. In another study by Senol et al., the lipid peroxidation and IL-1 β values were decreased by selenium treatments, whereas plasma levels of IL-4, brain cortex GSH, total antioxidant status, and Vitamin C and E values were increased by administrating selenium.[13] Yeo and Kang also indicated that selenite potentially inhibited H2O2-induced apoptosis of neural progenitor cells in TBI.[11] This in vivo protective function could be associated with inhibition of H2O2-induced ROS generation, cytochrome c release, and caspase-3 and -9 activation.
Considering significant effects of selenium use on critical ill conditions and its positive effects on diseases outcome, the lack of association between selenium use and outcome of TBIs might be related to using low dosages of selenium and considering short follow-up time. Thus, to ensure the beneficial effects of drug, further studies with administrating different selenium dosages and with longer follow-up times are essential.
Conclusion
This human trial study could not demonstrate beneficial effects of intravenous infusion of selenium in the improvement of outcomes in patients with acute TBI.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors wish to thank Rasoul-e-Akram Hospital, Clinical Research Development Center, Iran University of Medical Science, for technically supported implementation of the project.
References
- 1.Bruns J, Jr, Hauser WA. The epidemiology of traumatic brain injury: A review. Epilepsia. 2003;44(Suppl 10):2–10. doi: 10.1046/j.1528-1157.44.s10.3.x. [DOI] [PubMed] [Google Scholar]
- 2.Pearn ML, Niesman IR, Egawa J, Sawada A, Almenar-Queralt A, Shah SB, et al. Pathophysiology Associated with Traumatic Brain Injury: Current Treatments and Potential Novel Therapeutics. Cell Mol Neurobiol. 2016 doi: 10.1007/s10571-016-0400-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prins M, Greco T, Alexander D, Giza CC. The pathophysiology of traumatic brain injury at a glance. Dis Model Mech. 2013;6:1307–15. doi: 10.1242/dmm.011585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greve MW, Zink BJ. Pathophysiology of traumatic brain injury. Mt Sinai J Med. 2009;76:97–104. doi: 10.1002/msj.20104. [DOI] [PubMed] [Google Scholar]
- 5.McAllister TW. Neurobiological consequences of traumatic brain injury. Dialogues Clin Neurosci. 2011;13:287–300. doi: 10.31887/DCNS.2011.13.2/tmcallister. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rodríguez-Rodríguez A, Egea-Guerrero JJ, Murillo-Cabezas F, Carrillo-Vico A. Oxidative stress in traumatic brain injury. Curr Med Chem. 2014;21:1201–11. doi: 10.2174/0929867321666131217153310. [DOI] [PubMed] [Google Scholar]
- 7.Cornelius C, Crupi R, Calabrese V, Graziano A, Milone P, Pennisi G, et al. Traumatic brain injury: Oxidative stress and neuroprotection. Antioxid Redox Signal. 2013;19:836–53. doi: 10.1089/ars.2012.4981. [DOI] [PubMed] [Google Scholar]
- 8.Mendes Arent A, de Souza LF, Walz R, Dafre AL. Perspectives on molecular biomarkers of oxidative stress and antioxidant strategies in traumatic brain injury. Biomed Res Int 2014. 2014 doi: 10.1155/2014/723060. 723060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shen Q, Hiebert JB, Hartwell J, Thimmesch AR, Pierce JD. Systematic review of traumatic brain injury and the impact of antioxidant therapy on clinical outcomes. Worldviews Evid Based Nurs. 2016;13:380–9. doi: 10.1111/wvn.12167. [DOI] [PubMed] [Google Scholar]
- 10.Naziroglu M, Senol N, Ghazizadeh V, Yürüker V. Neuroprotection induced by N-acetylcysteine and selenium against traumatic brain injury-induced apoptosis and calcium entry in hippocampus of rat. Cell Mol Neurobiol. 2014;34:895–903. doi: 10.1007/s10571-014-0069-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yeo JE, Kang SK. Selenium effectively inhibits ROS-mediated apoptotic neural precursor cell death in vitro and in vivo in traumatic brain injury. Biochim Biophys Acta. 2007;1772:1199–210. doi: 10.1016/j.bbadis.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 12.Uttara B, Singh AV, Zamboni P, Mahajan RT. Oxidative stress and neurodegenerative diseases: A review of upstream and downstream antioxidant therapeutic options. Curr Neuropharmacol. 2009;7:65–74. doi: 10.2174/157015909787602823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Senol N, Naziroglu M, Yürüker V. N-acetylcysteine and selenium modulate oxidative stress, antioxidant vitamin and cytokine values in traumatic brain injury-induced rats. Neurochem Res. 2014;39:685–92. doi: 10.1007/s11064-014-1255-9. [DOI] [PubMed] [Google Scholar]


