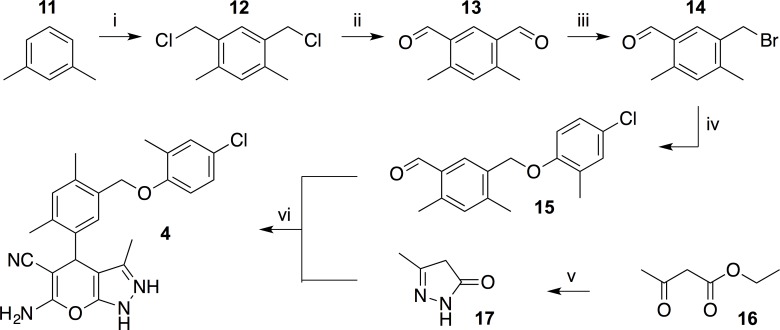
Fig 3. Reaction pathway for the synthesis of dihydropyrano[2,3-c]pyrazole 4.
(i) Parafomaldehyde, AcOH, conc. HCl (aq), 70°C, 48 h (37%); (ii) hexamethylenetetramine, EtOH, H2O, reflux, 16 h (37%); (iii) (a) NaBH4, EtOH, (b) HBr, AcOH; (iv) 4-chloro-2-methylphenol. K2CO3, CH3CN, 50°C, 3 h (99%); (v) hydrazine hydrate, EtOH, 0°C to 60°C, 3 h (90%); (vi) malonitrile, Et3N, EtOH, 80°C, 17 min (72%).