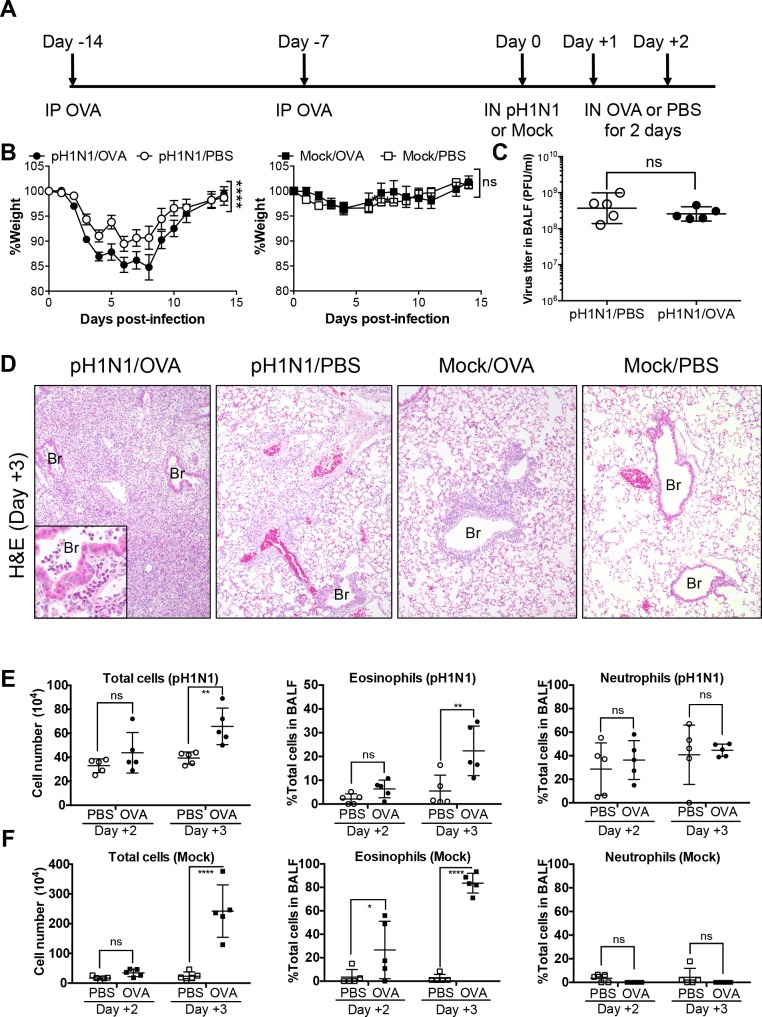
Fig 1. Influenza virus infection and subsequent induction of acute allergic airway responses by OVA in OVA-sensitized NC/Nga mice.
(A) Schematic representation of the protocol for OVA sensitization, influenza virus infection, and OVA-induced allergic airway responses. NC/Nga mice were sensitized twice by IP injection of OVA on Day -14 and Day -7 and then infected with a sublethal dose of influenza virus A/H1N1pdm09 (pH1N1) or Mock allantoic fluid (Day 0). On the 2 days following infection, the mice were challenged again with OVA or PBS via the IN route (Day +1 and Day +2). (B) Weight loss was monitored after infection (20 mice in the pH1N1 infection groups and 10 mice in the Mock groups). Error bars represent the mean ± SEM. Weight change data were combined from three (pH1N1 infection groups) or two (Mock groups) independent experiments. (C) On Day 3 post-infection, virus titers in the BALF were measured in a plaque assay (5 mice/group). Data are expressed as scatter plots with the mean viral titer ± SD. (D) Representative lung tissue sections from pH1N1/OVA, pH1N1/PBS, Mock/OVA, and Mock/PBS mice on Day 3 post-infection (3 mice/group). Sections were stained with hematoxylin and eosin (H&E). Original magnification: ×10 or ×40 (inset). (E) Total cell counts and the proportion of inflammatory cells in the BALF form pH1N1/PBS and pH1N1/OVA mice assessed on Day 2 and Day 3 post-infection (5 mice/group). Data are expressed as scatter plots with the mean ± SD. (F) Total cell counts and the proportion of inflammatory cells in the BALF form Mock/PBS and Mock/OVA mice assessed on Day 2 and Day 3 post-infection (5 mice/group). Data are expressed as scatter plots with the mean ± SD. *p<0.05, **p<0.01, ***p<0.001, and ****p<0.0001 (two-way ANOVA or Mann-Whitney U test). IP, intraperitoneal; IN, intranasal; BALF, bronchoalveolar lavage fluid; ns, not significant; Br, Bronchiole. The experiments were repeated independently at least twice.