Figure 7.
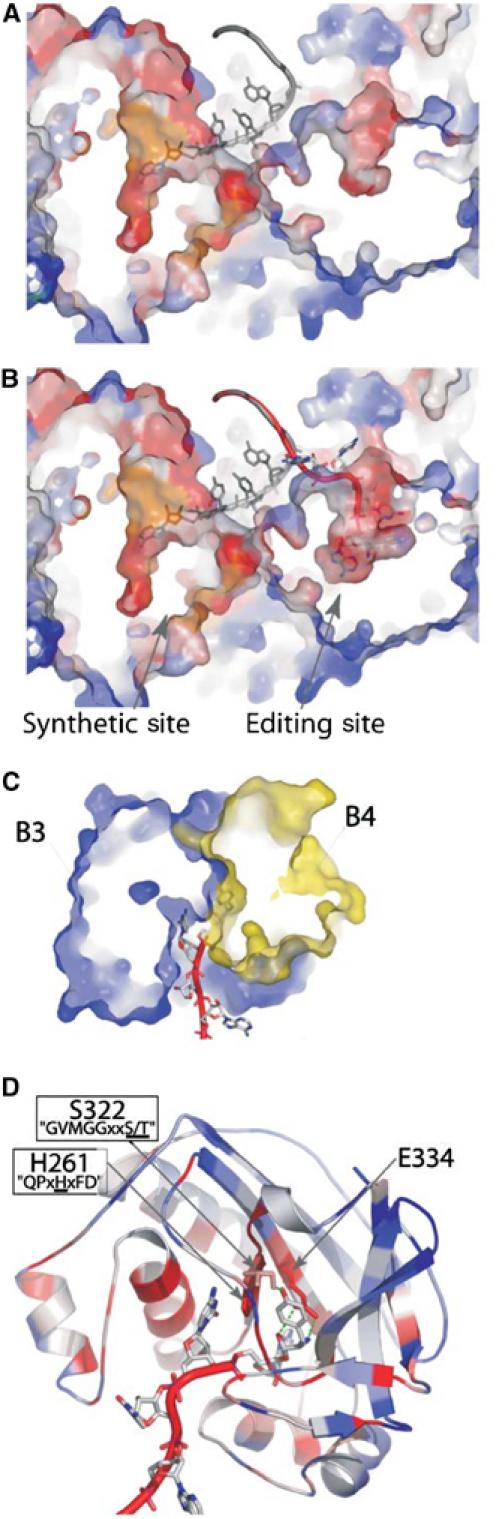
Model of the post-transfer editing site of PheRS. (A) Cross section of T. thermophilus PheRS in complex with tRNAPhe (1EIY). The CCA moiety of tRNAPhe (grey) is bound into the synthetic site of the α subunit of the enzyme. (B) Model for Tyr-tRNAPhe (red) binding in the editing site of the B3/B4 domain of the β subunit (see text, geometry of the model was optimized with DSviewer pro 5.0 (Accelrys)). tRNAPhe (grey) as found in the original structure is superimposed. (C) Cross section of the model of the B3/B4 domain bound to Tyr-tRNAPhe. The editing site is localized at the interface of domains B3 (in blue) and B4 (in yellow). (D) Ribbon representation of the model of the B3/B4 domain bound to Tyr-tRNAPhe. A76-Tyr is maintained between the two conserved motifs ‘GVMGGxxS/T' and ‘QPxHxFD'. Conserved residues in close contact with the Tyr moiety are displayed. Except for tRNAs and part (C), colors represent the percentage of identity for each position in an alignment of 103 eubacterial PheRSs (see Figure 3).
