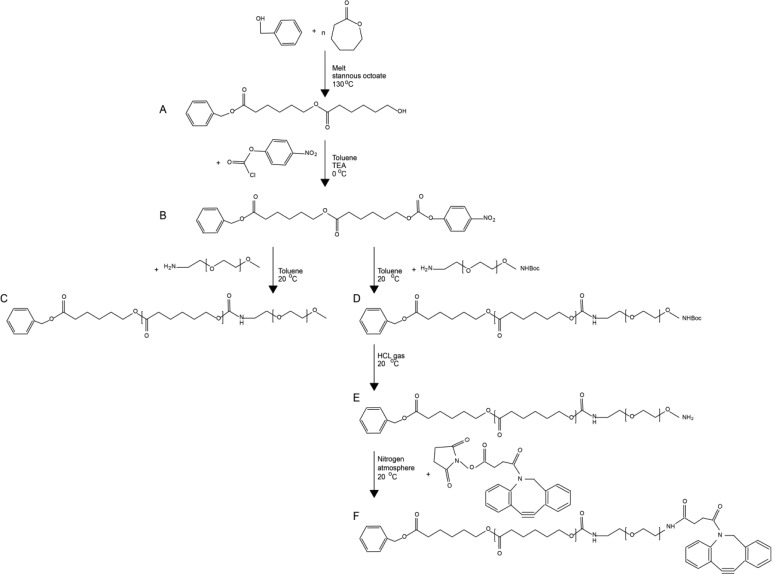
Figure 7.
Synthesis routes for ben-PCL7-mPEG2000 and ben-PCL7-mPEG2000-DBCO. Synthesis of both molecules starts with (A) ring opening polymerization of ε-caprolactone with benzyl alcohol which affords ben-PCL7-OH and (B) activation of the hydroxyl end group with p-nitrophenylchloroformate. Then for synthesis of amphiphilic ben-PCL7-mPEG2000, (C) mPEG2000-NH2 is coupled to the activated ben-PCL7-PNC. For synthesis of clickable ben-PCL7-mPEG2000-DBCO, (D) NH2-PEG2000-NHBoc is coupled to the activated ben-PCL7-PNC, (E) the Boc protection group is removed with HCl gas, and (F) NHS-DBCO is conjugated to ben-PCL7-mPEG2000-NH2.