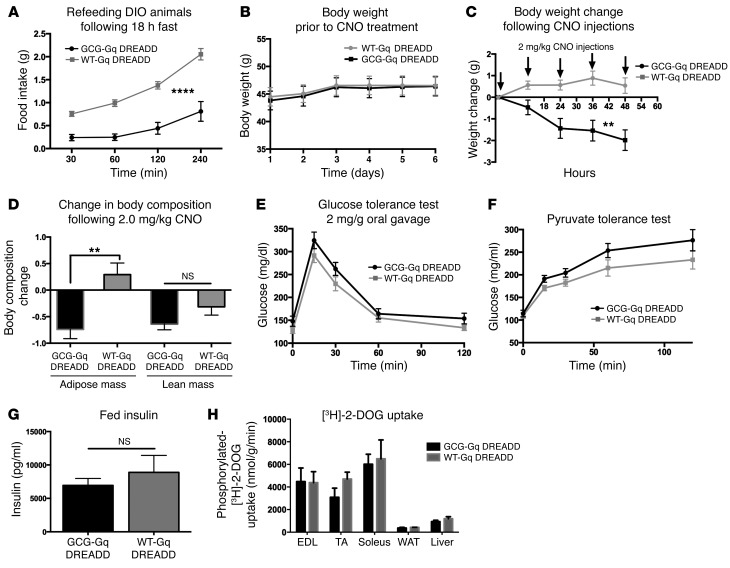
Figure 7. Anorexigenic effect of GCG neuronal stimulation is enhanced in DIO Gcg-Cre mice.
n = 6–7 control and GCG-Gq DREADD mice were fed HCD (Teklad TD88137) ad libitum for 5 months prior to testing. (A) CNO stimulation reduced food intake following an 18-hour fast compared with control CNO-injected littermates (2-way repeated measures ANOVA, significant effects of time [F3,33 = 72.91, ****P < 0.0001] and CNO treatment [F1,11 = 36.04, ****P < 0.0001]). (B) Prior to testing the effects of CNO injection on body weight, control and GCG-Gq DREADD mice exhibited no difference in mass (2-way repeated measures ANOVA, F1,11 = 0.02455, P = 0.8783). (C) Regular injections of CNO spaced 12 hours apart significantly reduced body weight in the GCG-Gq DREADD animals but had no effect on controls (2-way repeated measures ANOVA, significant effects of time [F4,44 = 5.02, **P = 0.002] and CNO [F1,11 = 14.59, **P = 0.0028]). (D) Fat mass was reduced (t test, t = 3.76, **P = 0.0032), while no change in lean mass (t test, P = 0.11) was seen. (E and F) No effect of CNO injection on glucose homeostasis was observed. (E) Two-way repeated measures ANOVA, significant effect of time (F4,44 = 42.82, P < 0.0001), no effect of CNO treatment (F1,11 = 4.182, P = 0.655). (F) Two-way repeated measures ANOVA, significant effect of time (F4,44 = 86.73, P < 0.0001), no effect of CNO treatment (F1,11 =4.447, P = 0.0587). No effect of CNO was observed on fed insulin levels (G, t test t = 0.758, P = 0.464) or on glucose uptake (H, n = 3 mice per group, t test for each tissue sourced from CNO-treated WT and GCG-Gq DREADD animals, EDL t = 0.058, P = 0.958; TA t = 1.604, P = 0.1840; soleus t = 0.2589, P = 0.8085; WAT t = 0.8964, P = 0.4207; liver t = 1.200, P = 0.2964).