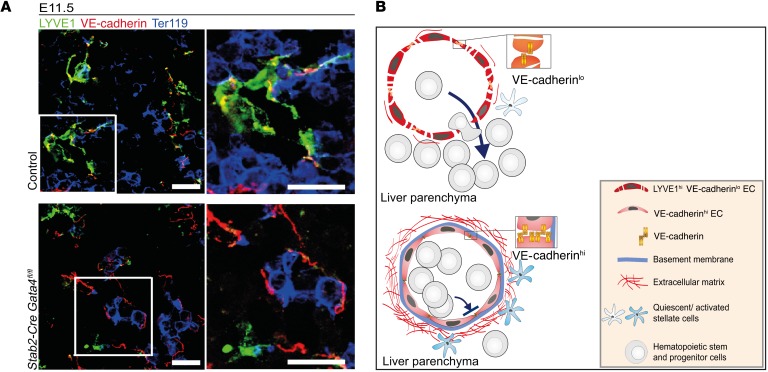
Figure 13. Endothelial GATA4 expression preserves liver sinusoidal endothelial identity with high junctional permeability and limited ECM deposition permissive for hepatic stem cell colonization and unperturbed liver development and erythropoiesis.
(A) Co-IF of LYVE1, Ter119, and VE-cadherin in the liver of Stab2-Cre Gata4fl/fl embryos at E11.5 (n = 3). Scale bars: 20 μm. (B) GATA4 serves as the master regulator of the fetal hepatic vascular niche. During normal development, GATA4-dependent gene expression cascades in ECs promote discontinuous sinusoidal endothelial differentiation featuring weak cell-cell contacts, lack of a basement membrane, and low levels of ECM deposition, resulting in an antifibrotic perivascular microenvironment. The hepatic sinusoidal microvasculature supports transmigration of hematopoietic stem and progenitor cells into the liver parenchyma, a process indispensable for liver development. In the absence of GATA4, hepatic sinusoidal microvessels undergo continuous transdifferentiation/capillarization, including basement membrane formation followed by ECM deposition and stellate cell activation, and lethal impairment of hematopoiesis via stabilization of VE-cadherin+ junctions.