Abstract
There are many causes of gastrointestinal bleeding (GIB) in children, and this condition is not rare, having a reported incidence of 6.4%. Causes vary with age, but show considerable overlap; moreover, while many of the causes in the pediatric population are similar to those in adults, some lesions are unique to children. The diagnostic approach for pediatric GIB includes definition of the etiology, localization of the bleeding site and determination of the severity of bleeding; timely and accurate diagnosis is necessary to reduce morbidity and mortality. To assist medical care providers in the evaluation and management of children with GIB, the “Gastro-Ped Bleed Team” of the Italian Society of Pediatric Gastroenterology, Hepatology and Nutrition (SIGENP) carried out a systematic search on MEDLINE via PubMed (http://www.ncbi.nlm.nih.gov/pubmed/) to identify all articles published in English from January 1990 to 2016; the following key words were used to conduct the electronic search: “upper GIB” and “pediatric” [all fields]; “lower GIB” and “pediatric” [all fields]; “obscure GIB” and “pediatric” [all fields]; “GIB” and “endoscopy” [all fields]; “GIB” and “therapy” [all fields]. The identified publications included articles describing randomized controlled trials, reviews, case reports, cohort studies, case-control studies and observational studies. References from the pertinent articles were also reviewed. This paper expresses a position statement of SIGENP that can have an immediate impact on clinical practice and for which sufficient evidence is not available in literature. The experts participating in this effort were selected according to their expertise and professional qualifications.
Keywords: Gastrointestinal bleeding, Endoscopy, Lower gastrointestinal bleeding, Upper gastrointestinal bleeding, Pediatric
Core tip: This review provides a practical diagnostic guide for clinicians for the diagnosis and management of gastrointestinal bleeding (GIB) in children. Clinical presentation can be variable and bleeding can occur in any area of the gastrointestinal tract. The differential diagnosis is important to define the sequence of management. Upper endoscopy and colonoscopy are the mainstay of initial investigations. Best outcomes are possible by a multidisciplinary approach including clinicians with skills in pediatric gastroenterology, radiology and surgery. For cases of major GIB, stabilization of the patient’s condition precludes any diagnostic examination.
INTRODUCTION
Gastrointestinal bleeding (GIB) is a common condition in children and can occur in any area of the gastrointestinal tract, from the mouth to the anus. Fortunately, mortality for acute gastrointestinal bleeding (AGIB) is low in the pediatric population.
Over the last 10 years, there have been a number of improvements in diagnosis and management of GIB in general. Increased involvement has been seen in the management of AGIB and resuscitation and in the correct usage of diagnostic and therapeutic endoscopy. In addition, GIB cases have benefited from advances in diagnostic and therapeutic radiology techniques and equipment, as well as development of more selective and less invasive surgical approaches and of more efficacious, tolerable and safe ulcer-healing drugs. These changes have modified the diagnostic and treatment strategies for patients presenting with non-variceal and variceal upper GIB (UGIB) and those with colonic bleeding.
The major objectives of GIB management are to reduce mortality and the need for major surgery. A secondary objective is to prevent unnecessary hospital admission for patients presenting with minor or self-limited bleeding. This position paper provides recommendations based on current evidence for best practice in the management of acute UGIB and lower GIB (LGIB) in children; management of patients over the age of 18 is not covered by this statement. This statement will be of interest for generalist and specialized pediatricians, as well as general medical professionals who may encounter pediatric patients among their patient population, such as acute physicians, gastroenterologists, gastrointestinal surgeons, endoscopists, pharmacists, anesthesiologists and nurses.
The statement presented herein resulted from a first-phase systematic literature search and review by experts comprising the “Gastro-Ped Bleed Team” of the Italian Society of Pediatric Gastroenterology, Hepatology and Nutrition (SIGENP). The preliminary draft was first circulated among the panel and a subsequent meeting was held, in which a consensus was reached on the points touched, resulting in the final statement that is presented herein. It is important to note that this position paper is not intended to be construed or to serve as a standard of care. Standards of care are determined on the basis of all clinical data available for an individual case and are subject to change as scientific knowledge and technology advances and patterns of care evolve.
DEFINITIONS
UGIB is that originating proximal to the ligament of Treitz, and, in practice, from the esophagus, stomach and duodenum. LGIB is defined as bleeding distal to the ligament of Treitz. Hematemesis (and coffee-ground vomitus) is vomiting of blood from the upper gastrointestinal tract or, occasionally, after swallowing blood from a source in the nasopharynx[1]. Bright red hematemesis usually implies active hemorrhage from the esophagus, stomach or duodenum. Coffee-ground vomitus refers to the vomiting of black material, which is assumed to be blood. Melena is the passage of black tarry stools, usually due to acute UGIB but occasionally from bleeding within the small bowel or right side of the colon. Hematochezia is the passage of fresh or altered blood via rectum, usually due to colonic bleeding[2].
Shock is circulatory insufficiency, resulting in inadequate oxygen delivery that leads to global hypoperfusion and tissue hypoxia; in the context of GIB, shock is most likely to be hypovolemic (due to the inadequate circulating volume resulting from acute blood loss). Varices are abnormal distended veins, most frequently occurring in the esophagus (esophageal varices) and less frequently in the stomach (gastric varices) or other sites (ectopic varices), and usually occurring as a consequence of liver disease; variceal bleeding is characteristically severe and may be life-threatening[3]. Endoscopy is the visualization of the inside of the gastrointestinal tract accomplished by means of videoscope. Examination of the upper gastrointestinal tract (esophagus, stomach and duodenum) is known as gastroscopy or upper gastrointestinal endoscopy. Examination of the colon (large bowel) is referred to as colonoscopy. A list of definitions is provided in Table 1.
Table 1.
Definitions
| Upper gastrointestinal bleeding | GI bleeding originating proximal to the ligament of Treitz (esophagus, stomach and duodenum) |
| Lower gastrointestinal bleeding | GI bleeding originating distal to the ligament of Treitz (small bowel and colon) |
| Occult gastrointestinal bleeding | GI bleeding that is not visible to the patient or physician, resulting in either a positive fecal occult blood test or iron-deficiency anemia |
| Hematemesis | Vomiting of blood or coffee-ground-like material |
| Hematochezia | Passage of fresh blood per anus |
| Melena | Passage of black, tarry stools per anus |
GI: Gastrointestinal.
UGIB
In children, UGIB is an uncommon but potentially serious, life-threatening clinical condition. From an anatomical perspective, the UGIB tract encompasses the gastrointestinal region from the esophagus to the ligament of Treitz[4]. A study by Cleveland et al[5], involving 167 patients, showed the common signs and symptoms of UGIB at presentation to be hematemesis (73%), melena (21%) and coffee-ground emesis (6%); however, patients may also experience epigastric pain, abdominal tenderness or dizziness.
The worldwide mortality rate for UGIB in children can range from 5% to 15%, reflecting the diverse populations that differentially experience conditions associated with UGIB, such as acute variceal hemorrhage[4,6]. The causes of UGIB have been classified based upon variceal bleeding and non-variceal bleeding (Table 2)[7]. Case series reported from Asia and developing countries show a higher incidence of variceal bleeding[8].
Table 2.
Causes of upper gastrointestinal bleeding based on age
| Infants | 2-5 years | Older | |
| Esophagus | Esophagitis | Esophagitis | |
| Esophageal varices | Mallory-Weiss syndrome | ||
| Mallory-Weiss syndrome | Esophageal varices | ||
| Stomach | Gastritis from stress | Gastritis | Dieulafoy lesion |
| Gastric ulcer | PHG | ||
| Gastric varices | Hemobilia | ||
| Duodenum | Duodenitis | ||
| Duodenal ulcer | |||
| Variable location | Vitamin K deficiency | Caustic ingestions | Polyps |
| Sepsis | Foreign bodies | Crohn’s disease | |
| Trauma (NG tubes) | NSAIDs use | Telangiectasia | |
| CMPA | Aortoenteric fistula | ||
| Coagulation disorders | |||
| Caustic ingestions | |||
| Foreign bodies | |||
| NSAIDs use |
NG: Nasogastric; CMPA: Cow's milk protein allergy; NSAIDs: Non-steroidal anti-inflammatory drugs; PHG: Portal hypertensive gastropathy.
The etiology of UGIB can be categorized by age groups, but causative disorders overlap considerably between these[4]. In newborns, the predominant causes include coagulation disorders, such as vitamin K deficiency, cow’s milk protein allergy (CMPA)[9], stress-related gastritis, sepsis, and trauma from placement of nasogastric tubes. In infants (1 mo to 1 year of age), the most prevalent causes are caustic ingestions, duplication cysts, foreign body ingestion, and medication-induced. In toddlers and young children (1 year to 5 years of age), causes include erosive esophagitis, gastritis, caustic ingestions, peptic ulcer bleeding, varices, and vomiting-induced bleeding (e.g., from a Mallory-Weiss tear). In children and adolescents (ages 5 years to 18 years), bleeding can arise from coagulation disorders, gastritis, Dieulafoy lesions (angiodysplasia), erosive esophagitis, peptic ulcer disease, caustic ingestions, and vomiting-induced bleeding[10].
Crohn’s disease is an uncommon cause of UGIB in the pediatric population[11]. Certain foods may create confusion by mimicking the appearance of blood in vomitus [e.g., artificial (red) food-coloring, fruit-flavored drinks, fruit juices, and beets]. All findings suspicious of blood in vomitus should be clinically investigated further[12].
The current diagnostic approach for pediatric UGIB has been mostly extrapolated from studies of adults; the key points are extensive history-taking and examination, laboratory evaluations, and diagnostic procedures[13]. Maternal sources of blood include ingestion of blood during the delivery or from cracked nipples during breastfeeding; infants who ingest maternal blood may present with hematemesis or melena[4]. Historical information includes the presence of abdominal pain, coffee-ground-like emesis, dysphagia, black and tarry stools, bright red blood via rectum, hematemesis, and chest pain. In addition, drug use should be elicited, especially any previous use of non-steroidal anti-inflammatory drugs (NSAIDs), aspirin and/or corticosteroids[14]. The physician should also ascertain a history of peptic ulcer bleeding or surgery, as well as any previous episodes of UGIB and previous history of umbilical catheterization[15].
In newborns with suspected UGIB, an alkali denaturation test (i.e., the Apt-Downey test) can differentiate neonatal blood from maternal blood. Gastric lavage via nasogastric tube can improve the accuracy of endoscopy[16,17]. Upper endoscopy is the test of choice for evaluating hematemesis. The goals of endoscopy in UGIB are to identify the site of bleeding and to facilitate initiation of an appropriate therapeutic approach when indicated[5].
A flowchart of the diagnostic approach of UGIB is provided in Figure 1. In summary, UGIB refers to bleeding above the ligament of Treitz and the priority of achieving a differential diagnosis addresses both the clinical presentation and the age of the patient.
Figure 1.
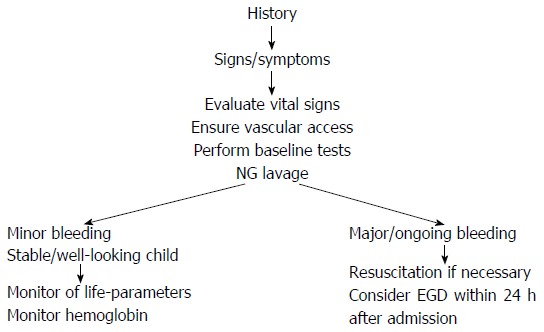
Diagnostic approach of upper gastrointestinal bleeding in infants and children. NG: Nasogastric; EGD: Upper endoscopy.
LGIB
LGIB in children is a common clinical problem; indeed, it is reportedly the presenting complaint for approximately 0.3% of children in the emergency department[18]. In most cases, the bleeding is self-limiting, with the majority (80%) of LGIB cases in the emergency department undergoing routine discharge[19]. However, conditions such as Meckel’s diverticulum, melena by variceal hemorrhages, acute intestinal obstruction or severe attack of ulcerative colitis often present with life-threatening GIB.
The etiology of LGIB is very different between children and adults, and its incidence is age-dependent. The main causes of LGIB in adults are colorectal cancer, colorectal polyps, anorectal disease and inflammatory bowel diseases (IBDs); in children, colorectal polyps, chronic colitis and perianal lesions are the main causes[20]. In infants, allergic colitis and anorectal fissures represent the most common causes, while infectious enteritis and anorectal fissures are the most common causes in older children[21] (Table 3). In young infants (< 1 year of age), the most likely cause of hematochezia with or (more often) without diarrhea is the so-called allergic colitis; although CMPA is usually suspected, the etiology is often uncertain. In breastfed infants, without anemia, who are growing well, hematochezia is usually a benign self-limiting disorder, and a maternal milk-free diet is not necessarily indicated[22].
Table 3.
Causes of lower gastrointestinal bleeding based on age
| Infants | 2-5 years | Older |
| Non-specific colitis | Polyps | Anal fissure |
| Anal fissure | Anal fissure | Infectious Enterocolitis |
| Milk allergy | Infectious enterocolitis | Polyps |
| Duplication of bowel | Intussusception | Inflammatory bowel disease |
| Volvulus | Meckel’s diverticulum | Lymphonodular hyperplasia |
| Hirschsprung’s disease | Henoch-Schonlein purpura | Henoch-Schonlein purpura |
| Necrotizing enterocolitis | Hemolytic-uremic syndrome | Angiodysplasia |
| Bleeding diathesis | Lymphonodular hyperplasia | Hemolytic-uremic syndrome |
| Angiodysplasia | Bleeding diathesis |
A valid approach to investigate the causes of LGIB is to classify it according to the child’s age, general appearance (ill or well), bleeding rate, and stool characteristics[23]. Meckel’s diverticulum should strongly be suspected, at any age, if bleeding is massive and accompanied by both bright and dark red stools. In ill infants, ischemic/surgical causes, such as mid-gut volvulus and intussusception, should be suspected. In older children, other serious medical causes, such as severe attack of ulcerative colitis, Henoch-Schonlein purpura or hemolytic-uremic syndrome, might be the cause of bleeding[24].
In cases of severe LGIB, especially when melena is present or the patient is hemodynamically unstable, the source of bleeding may include the upper gastrointestinal region[25]. In cases with bloody diarrhea that is persistent (> 7 d), recurrent or severe (> 7 bloody stools/d), the child should be seen by a pediatric gastroenterologist with indication to endoscopy. Rectal bleeding with normal stool pattern is suggestive of the presence of juvenile polyp, nodular lymphoid hyperplasia or eosinophilic colitis, as well as IBD and, rarely, vascular malformations.
In a retrospective cross-sectional study, de Ridder et al[26] reported data of 137 children undergoing colonoscopy for rectal bleeding (mean duration of 28 wk). The diagnosis rate for first colonoscopy (IBD and colonic polyps) was 80%. No abnormalities were found in 20.4% of the patients, either by colonoscopy or histopathology, and the final diagnosis for these cases was self-limited GIB.
Constipation is commonly associated with the presence of anal fissure and pain on defecation. Visual inspection of the perianal area as well as digital rectal examination are mandatory to detect the possibility of anal fissure, streptococcal cryptitis or rectal polyp. Endoscopy within 6 h after the first evaluation is rarely needed; in cases of severe colitis, a rapid diagnosis and histological evaluation may necessitate a proctosigmoidoscopy without bowel cleansing[23].
In conclusion, the main priority for the physician in evaluating a patient with LGIB is to identify those patients in whom bleeding is secondary to intestinal obstruction or surgical causes. An algorithm of the diagnostic approach of LGIB is presented in Figure 2.
Figure 2.
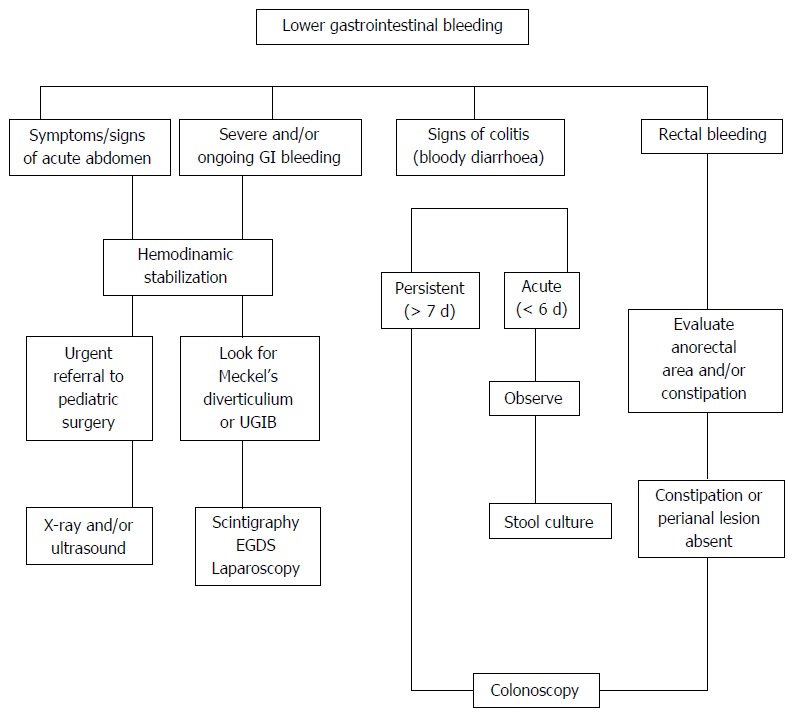
Diagnostic approach of lower gastrointestinal bleeding in infants and children. UGIB: Upper gastrointestinal bleeding; GI: Gastrointestinal.
PRIMARY CLINICAL MANAGEMENT
Stabilization of general conditions should precede any instrumental investigation (usually endoscopy) for children with GIB. The best clinical indicator of blood loss is orthostatic changes in heart rate and blood pressure; defined as an increase in pulse rate by 20 beats/min or a decrease in systolic blood pressure of 10 mmHg or more upon moving the patient from supine to sitting position. For any other emergency situation, the first priority should be to assess the airways, breathing and circulation of the patient[5].
The most important aspect of the initial GIB evaluation is to determine the degree and rapidity of blood loss, and any risk factors (i.e., coagulopathy, sepsis, trauma) or associated signs (i.e., purpuric lesions, hepatosplenomegaly, jaundice, cutaneous hemangiomas, eczema)[7]. In the case of a child with no clinical impairment, it is sufficient to ensure vascular access and perform baseline tests (i.e., blood count and group, liver and kidney function, blood coagulation) as well as a pre-anesthesia examination. For cases of UGIB, nasogastric aspiration and saline lavage are indicated to confirm the presence of intragastric blood[27], to determine the rate of gross bleeding, to check for ongoing or recurrent bleeding, to clear the gastric field for subsequent endoscopic visualization, to prevent aspiration of gastric contents and to prevent hepatic encephalopathy in patients with cirrhosis. Parenteral vitamin K (1-2 mg/dose) should be administered empirically to infants, even when results of coagulation are pending. The finding of coagulopathy with an international normalized ratio > 1.5 or abnormal partial thromboplastin time should be corrected by administration of fresh frozen plasma (10 mL/kg initially); cryoprecipitate administration may be tried in the presence of severe coagulopathy, especially if the volume of fluid has to be restricted.
In conclusion, supportive measures with stabilization of hemodynamic status, correction of any coagulation or platelet abnormalities are necessary before diagnostic procedures are undertaken.
OBSCURE GASTROINTESTINAL BLEEDING
Obscure gastrointestinal bleeding (OGIB) is defined as bleeding of unknown origin that persists or recurs after negative findings on initial evaluation using bidirectional endoscopy[28]. It can be classified as overt or occult, based on presence or absence of clinically-evident bleeding. Obscure-occult bleeding is generally determined by a positive fecal occult blood test result and/or iron-deficiency anemia[29]. Chronic occult GIB may occur anywhere in the gastrointestinal tract-from the oral cavity to the anorectum. In most cases, the site is identified by upper endoscopy and ileocolonoscopy. Causes depend on age of presentation (i.e., infants, children, adolescents) and location of gastrointestinal tract bleeding. OGIB may be active, as with melena, hematochezia or hematemesis, or it may be inactive, showing intermittent bleeding.
Similar to data from adult patients[30], OGIB accounts for 5% of all pediatric cases of GIB, including both acute overt and chronic occult types of blood loss. In approximately 75% of OGIB cases, the lesions are detected in the small bowel (mid-GIB) distal to Vater’s papilla and reaching as far as the terminal ileum. The source of mid-GIB is related to age, with children showing a greater likeliness of having small intestinal polyps, Meckel’s diverticulum, vascular malformations, Crohn’s disease, anastomotic ulcers and intestinal duplications[31].
Diagnostic approaches for OGIB, after negative endoscopy and colonoscopy, can require small bowel endoscopic investigation by video capsule endoscopy (VCE). Balloon-assisted enteroscopy (BAE), with single or double-balloon enteroscopy (DBE), is the second-line technique, having the advantage of therapeutic as well as diagnostic properties. The diagnostic yield is very good (70%-100%), and is significantly higher when BAE is performed after a positive VCE. In a recent pediatric study of 117 children treated with DBE (total of 257 procedures), Yokoyama et al[32] found the greatest indication to be OGIB (61.9%) and a low incidence of complications (5.4%), regardless of the associated therapeutic procedures.
Intraoperative enteroscopy, involving insertion of an endoscope through an incision in the mid-small intestine, is currently reserved as a last option, or if small intestinal endoscopy cannot be successfully performed. Laparoscopy and exploratory laparotomy remain important alternative diagnostic tools, for when other measures cannot identify a bleeding source in selected patients[33].
In conclusion, it is reasonable to perform both upper endoscopy and colonoscopy in a patient with OGIB (overt or occult) to identify pathological processes that can explain symptoms or iron-deficiency anemia.
IMAGING STUDIES
Radiological imaging has played an increasingly important role in the diagnosis and management of GIB over the past 30 years. Magnetic resonance imaging has emerged as key pediatric imaging modality, preferred for its lack of ionizing radiation; it is particularly suitable for studying small bowel pathologies, and is currently the first-line modality for such. The exact source of GIB may be localized by means of nuclear scintigraphy, as well as selective angiography. In general, examination by imaging is most commonly requested after negative endoscopy results, or for indeterminate causes or locations of bleeding.
The role of interventional radiology has also increased over the past years for the treatment of gastrointestinal hemorrhage, especially in very ill patients who are poor surgical candidates. Nuclear scintigraphy is a sensitive method for detecting GIB (used at a rate of 0.1 mL/min) and the method is more sensitive, but less specific, than angiography[34]. Although arteriographic diagnosis and therapy have been reviewed extensively in the literature describing adult cases, few experiences in children have been reported. In one published pediatric study, which involved 27 children, arteriography had an overall positive diagnostic rate of 64% and a false-negative rate of 36%. In AGIB, the diagnosis was correct in 71% and falsely negative in 29%, while in chronic or recurrent GIB, it was correct in 55% and falsely negative in 45%[35].
The only angiographic sign that is 100% diagnostic for AGIB is contrast extravasation in the intestinal lumen. However, other angiographic signs can be useful in evaluation of some of the more common pediatric pathologies that cause GIB. One of the main advantages of angiographic diagnosis of GIB is the ability to perform transcatheter treatment after the bleeding site has been located. The two main transcatheter therapies are intraarterial vasopressin infusion and embolization. The most serious complication related to the technique is bowel infarction. Hongsakul et al[36] reported the risk factors as being failure to achieve hemostasis, hemoglobin concentration, coagulopathy, UGIB, contrast extravasation, and > 1 embolized vessel.
THERAPY
The pharmacological treatment approach to UGIB and LGIB currently includes 3 classes of drugs: acid suppression drugs, vasoactive drugs, and non-selective β-blockers (NSBBs).
Acid suppression drugs
The proton pump inhibitors (PPIs) have shown benefit in treatment of ulcer-bleeding or UGIB patients and to be superior to the H2-antagonist. There are no differences between the 5 available PPIs: esomeprazole, lansoprazole, omeprazole, pantoprazole and rabeprazole. The recommended administration route is intravenous, as a 1-h infusion at a dose of 1-3 mg/kg to maintain 24-h gastric pH > 6 in active bleeding.
Dosing in children has been extrapolated from the adult literature; although, the available data suggest faster drug clearance and significant interindividual variability in pediatric patients. A meta-analysis of an adult population showed that PPI treatment, with or without endoscopic therapy, compared with placebo or an H2 receptor antagonist, reduced the risk of rebleeding and the need for surgery, but did not affect mortality[37]. Several studies showed that the rate of rebleeding, requirement of blood transfusion, and duration of hospital stay were less in PPI-treated patients[38]. Moreover, PPIs were shown to be effective in the treatment of GIB in children that had developed due to NSAIDs administration[39].
Vasoactive drugs
Vasoactive treatment should be administered as soon as possible when portal hypertension is the suspected cause of GIB. These medications are reported to stop bleeding in 75%-80% of cases[40]. Three vasoactive drugs (terlipressin, somatostatin, and octreotide) control variceal bleeding by reducing portal blood flow and portal pressure[41,42].
Terlipressin has an important systemic vasoconstrictor effect, which is more noticeable on the splanchnic arteries, causing an increase in systemic vascular resistance and arterial pressure as well as a significant (approximately 20%) and sustained (up to 4 h) decrease in portal vein pressure and flux[43,44]. Several randomized trials and meta-analyses have suggested that terlipressin provides a survival benefit, compared to placebo, to patients with variceal bleeding[45,46]. In adults, terlipressin can be considered as the first choice, with somatostatin or octreotide as the second choice. However, many studies that have compared the clinical efficacies of different types of vasoactive drugs, each administered as monotherapy, have found no differences in mortality rates. Studies in pediatric populations have yet to show the potential superiority of terlipressin over other vasoactive agents; however, Erkek et al[47] reported a single-child experience of its use for successful management of severe non-variceal UGIB. Studies have shown that terlipressin has a very good safety profile, compared to vasopressin, although adverse events such as hyponatremia and seizure have been described in children (thus, necessitating monitoring of sodium levels)[48].
Octeotride is a synthetic derivative of somatostatin. It produces selective splanchnic vasoconstriction and decreases portal inflow, thereby indirectly reducing variceal blood flow. In children, intravenously-administered octreotide is effective in decreasing AGIB. Studies of pediatric populations have demonstrated octeotride to be effective at dosages of 2-5 mcg/kg per hour administered by continuous infusion[49], and that initiation with a 1-h bolus may be needed, and to continue the infusion for at least 5 d in patients at risk of rebleeding seems an appropriate and rational choice[50]. However, there is limited evidence regarding the efficacy and safety of octreotide for chronic GIB in children.
NSBBs
NSBBs, such as propranolol, nadolol and carvedilol, have been widely studied in adults with portal hypertension and have been shown to reduce portal pressures by decreasing cardiac output and vasoconstricting the splanchnic vessels via blockade of ß-1 and ß-2 receptors; moreover, carvedilol seems to be more effective than the traditional NSBBs in reducing hepatic venous pressure gradient[51].
The pediatric experience described in the literature is limited to primary and secondary prophylaxis of variceal bleeding. No formal randomized controlled trials evaluating safety and efficacy of NSBBs in children have been published. In addition, appropriate dosing of β-blockers has not been established (currently ranging from 2 mg/kg per day to 8 mg/kg per day) and it is unknown whether targeting a change in heart rate of 25% is effective in reducing portal pressures and the related risk of variceal bleeding in children. Pediatric clinical data supporting use of NSBBs in preventing a first variceal bleed are also limited, likely because there is no indication to use β-blockers to prevent the formation of varices. NSBBs or endoscopic band ligation are recommended, according to the Baveno VI Consensus Workshop, for the prevention of first variceal bleeding of medium or large varices[52].
THERAPEUTIC ENDOSCOPY
The aim of therapeutic endoscopy is to stop bleeding and prevent rebleeding. Endoscopy-based diagnostic and therapeutic management is a goal of physicians treating GIB and should be performed when the patient has been stabilized, and preferably within 24 h of bleeding presentation[4,53]. Several techniques, including injection therapy, ablative therapy and mechanical therapy, have been recommended for AGIB, each of these depending on the bleeding characteristics, such as active, oozing or no visible bleeding vessel. In addition, each of these techniques have been adapted to upper and lower endoscopy, as well as to deep endoscopy.
Common therapies for GIB in adults and children include injection therapy with dilute epinephrine and sclerosants, ablation therapy (contact methods, such as thermocoagulation heater probe and electrocoagulation; non-contact methods, such as argon plasma coagulation) and mechanical therapy (such as with hemoclips and band ligation)[54,55]. Epinephrine injection arrests about 80% of non-variceal bleeding. Multiple adult meta-analyses have demonstrated that combination therapy (epinephrine injection in conjunction with clipping or ablation therapy) is superior to epinephrine alone in reducing the risk of rebleeding to about 10%[56,57]. The endoscopy laser and argon plasma coagulation methods can be effective therapies for GIB due to vascular abnormalities; indeed, using these, most bleeding from Mallory-Weiss tears stops spontaneously. For Dieulafoy lesions, which are very rare in children, endoscopy therapy is the first choice, using clipping, electrocautery, sclerosant injection, banding methods or laser. Endoclips are currently the preferred mechanical therapy for non-variceal GIB.
In management of acute variceal bleeding, endoscopic variceal ligation (EVL) is the treatment of choice; a meta-analysis confirmed the superiority of EVL compared with endoscopic sclerotherapy for major outcomes, such as recurrent bleeding, ulceration and stricture[58-60]. For therapeutic colonoscopy, adequate fasting time and appropriate bowel preparation is recommended to facilitate the visualization of mucosal lesions.
CONCLUSION
The diagnostic approach for GIB should include extensive history-taking and examination including laboratory evaluations and application of the available and most appropriate diagnostic procedures. Endoscopy is the method of choice for evaluating UGIB and LGIB, after stabilization and resuscitation, and within 24 h of presentation. The goals of endoscopy are to identify the site and etiology of the GIB, as well as to facilitate adequate treatment. Visual inspection of the perianal area and digital rectal examination should always be considered if a bright red blood coating is present on normal or hard stool.
In children, persistent or recurrent iron-deficiency anemia could be considered as a sign of OGIB, for which VCE is the first-line endoscopic investigation. Three vasoactive drugs (terlipressin, somatostatin, and octreotide) play a role in the control of variceal bleeding and all act by reducing portal blood flow and portal pressure. Endoscopy has a therapeutic role for polyps, ulcers, erosions, blue nevi, angiodysplasia, varices, strictures and scalloping.
ACKNOWLEDGMENTS
We gratefully acknowledge the support of the Italian Society of Pediatric Gastroenterology, Hepatology and Nutrition, without which the present study could not have completed.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Conflict-of-interest statement: All authors declare that they have no conflict of interest. The data presented, the statements made and the views expressed are solely the responsibility of the authors.
Peer-review started: September 19, 2016
First decision: December 19, 2016
Article in press: January 17, 2017
P- Reviewer: Jafari SA S- Editor: Yu J L- Editor: A E- Editor: Zhang FF
References
- 1.Treem WR. Gastrointestinal bleeding in children. Gastrointest Endosc Clin N Am. 1994;4:75–97. [PubMed] [Google Scholar]
- 2.Vinton NE. Gastrointestinal bleeding in infancy and childhood. Gastroenterol Clin North Am. 1994;23:93–122. [PubMed] [Google Scholar]
- 3.Hyams JS, Leichtner AM, Schwartz AN. Recent advances in diagnosis and treatment of gastrointestinal hemorrhage in infants and children. J Pediatr. 1985;106:1–9. doi: 10.1016/s0022-3476(85)80455-8. [DOI] [PubMed] [Google Scholar]
- 4.Owensby S, Taylor K, Wilkins T. Diagnosis and management of upper gastrointestinal bleeding in children. J Am Board Fam Med. 2015;28:134–145. doi: 10.3122/jabfm.2015.01.140153. [DOI] [PubMed] [Google Scholar]
- 5.Cleveland K, Ahmad N, Bishop P, Nowicki M. Upper gastrointestinal bleeding in children: an 11-year retrospective endoscopic investigation. World J Pediatr. 2012;8:123–128. doi: 10.1007/s12519-012-0350-8. [DOI] [PubMed] [Google Scholar]
- 6.Houben CH, Chiu PW, Lau JY, Lee KH, Ng EK, Tam YH, Yeung CK. Duodenal ulcers dominate acute upper gastrointestinal tract bleeding in childhood: a 10-year experience from Hong Kong. J Dig Dis. 2008;9:199–203. doi: 10.1111/j.1751-2980.2008.00346.x. [DOI] [PubMed] [Google Scholar]
- 7.Boyle JT. Gastrointestinal bleeding in infants and children. Pediatr Rev. 2008;29:39–52. doi: 10.1542/pir.29-2-39. [DOI] [PubMed] [Google Scholar]
- 8.Cochran EB, Phelps SJ, Tolley EA, Stidham GL. Prevalence of, and risk factors for, upper gastrointestinal tract bleeding in critically ill pediatric patients. Crit Care Med. 1992;20:1519–1523. doi: 10.1097/00003246-199211000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Zaher MM, Ahmed EM, Morsy AA. Case report: hematemesis could be an unusual presentation of cow’s milk protein allergy in children in Egypt. Egypt J Immunol. 2014;21:39–43. [PubMed] [Google Scholar]
- 10.Cox K, Ament ME. Upper gastrointestinal bleeding in children and adolescents. Pediatrics. 1979;63:408–413. [PubMed] [Google Scholar]
- 11.Pongprasobchai S, Nimitvilai S, Chasawat J, Manatsathit S. Upper gastrointestinal bleeding etiology score for predicting variceal and non-variceal bleeding. World J Gastroenterol. 2009;15:1099–1104. doi: 10.3748/wjg.15.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yachha SK, Khanduri A, Sharma BC, Kumar M. Gastrointestinal bleeding in children. J Gastroenterol Hepatol. 1996;11:903–907. [PubMed] [Google Scholar]
- 13.Rodgers BM. Upper gastrointestinal hemorrhage. Pediatr Rev. 1999;20:171–174. doi: 10.1542/pir.20-5-171. [DOI] [PubMed] [Google Scholar]
- 14.Wilkins T, Khan N, Nabh A, Schade RR. Diagnosis and management of upper gastrointestinal bleeding. Am Fam Physician. 2012;85:469–476. [PubMed] [Google Scholar]
- 15.Chawla S, Seth D, Mahajan P, Kamat D. Upper gastrointestinal bleeding in children. Clin Pediatr (Phila) 2007;46:16–21. doi: 10.1177/1084713806297151. [DOI] [PubMed] [Google Scholar]
- 16.Crook M. Haemoglobin in stools from neonates: measurement by a modified Apt-test. Med Lab Sci. 1991;48:346–347. [PubMed] [Google Scholar]
- 17.Urashima M, Toyoda S, Nakano T, Matsuda S, Kobayashi N, Kitajima H, Tokushige A, Horita H, Akatsuka J, Maekawa K. BUN/Cr ratio as an index of gastrointestinal bleeding mass in children. J Pediatr Gastroenterol Nutr. 1992;15:89–92. doi: 10.1097/00005176-199207000-00014. [DOI] [PubMed] [Google Scholar]
- 18.Osman D, Djibré M, Da Silva D, Goulenok C. Management by the intensivist of gastrointestinal bleeding in adults and children. Ann Intensive Care. 2012;2:46. doi: 10.1186/2110-5820-2-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Teach SJ, Fleisher GR. Rectal bleeding in the pediatric emergency department. Ann Emerg Med. 1994;23:1252–1258. doi: 10.1016/s0196-0644(94)70350-7. [DOI] [PubMed] [Google Scholar]
- 20.Pant C, Olyaee M, Sferra TJ, Gilroy R, Almadhoun O, Deshpande A. Emergency department visits for gastrointestinal bleeding in children: results from the Nationwide Emergency Department Sample 2006-2011. Curr Med Res Opin. 2015;31:347–351. doi: 10.1185/03007995.2014.986569. [DOI] [PubMed] [Google Scholar]
- 21.Bai Y, Peng J, Gao J, Zou DW, Li ZS. Epidemiology of lower gastrointestinal bleeding in China: single-center series and systematic analysis of Chinese literature with 53,951 patients. J Gastroenterol Hepatol. 2011;26:678–682. doi: 10.1111/j.1440-1746.2010.06586.x. [DOI] [PubMed] [Google Scholar]
- 22.Leung A, Wong AL. Lower gastrointestinal bleeding in children. Pediatr Emerg Care. 2002;18:319–323. doi: 10.1097/00006565-200208000-00022. [DOI] [PubMed] [Google Scholar]
- 23.Arvola T, Ruuska T, Keränen J, Hyöty H, Salminen S, Isolauri E. Rectal bleeding in infancy: clinical, allergological, and microbiological examination. Pediatrics. 2006;117:e760–e768. doi: 10.1542/peds.2005-1069. [DOI] [PubMed] [Google Scholar]
- 24.Balachandran B, Singhi S. Emergency management of lower gastrointestinal bleed in children. Indian J Pediatr. 2013;80:219–225. doi: 10.1007/s12098-012-0955-x. [DOI] [PubMed] [Google Scholar]
- 25.Murphy MS. Management of bloody diarrhoea in children in primary care. BMJ. 2008;336:1010–1015. doi: 10.1136/bmj.39542.440417.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Ridder L, van Lingen AV, Taminiau JA, Benninga MA. Rectal bleeding in children: endoscopic evaluation revisited. Eur J Gastroenterol Hepatol. 2007;19:317–320. doi: 10.1097/MEG.0b013e328080caa6. [DOI] [PubMed] [Google Scholar]
- 27.Singhi S, Jain P, Jayashree M, Lal S. Approach to a child with upper gastrointestinal bleeding. Indian J Pediatr. 2013;80:326–333. doi: 10.1007/s12098-013-0987-x. [DOI] [PubMed] [Google Scholar]
- 28.Raju GS, Gerson L, Das A, Lewis B. American Gastroenterological Association (AGA) Institute technical review on obscure gastrointestinal bleeding. Gastroenterology. 2007;133:1697–1717. doi: 10.1053/j.gastro.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 29.Neidich GA, Cole SR. Gastrointestinal bleeding. Pediatr Rev. 2014;35:243–253; quiz 254. doi: 10.1542/pir.35-6-243. [DOI] [PubMed] [Google Scholar]
- 30.Pennazio M, Spada C, Eliakim R, Keuchel M, May A, Mulder CJ, Rondonotti E, Adler SN, Albert J, Baltes P, et al. Small-bowel capsule endoscopy and device-assisted enteroscopy for diagnosis and treatment of small-bowel disorders: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy. 2015;47:352–376. doi: 10.1055/s-0034-1391855. [DOI] [PubMed] [Google Scholar]
- 31.Oliva S, Pennazio M, Cohen SA, Aloi M, Barabino A, Hassan C, Pession A, Lima M, Frediani S, Di Nardo G. Capsule endoscopy followed by single balloon enteroscopy in children with obscure gastrointestinal bleeding: a combined approach. Dig Liver Dis. 2015;47:125–130. doi: 10.1016/j.dld.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 32.Yokoyama K, Yano T, Kumagai H, Mizuta K, Ono S, Imagawa T, Yamamoto H, Yamagata T. Double-balloon Enteroscopy for Pediatric Patients: Evaluation of Safety and Efficacy in 257 Cases. J Pediatr Gastroenterol Nutr. 2016;63:34–40. doi: 10.1097/MPG.0000000000001048. [DOI] [PubMed] [Google Scholar]
- 33.Urs AN, Martinelli M, Rao P, Thomson MA. Diagnostic and therapeutic utility of double-balloon enteroscopy in children. J Pediatr Gastroenterol Nutr. 2014;58:204–212. doi: 10.1097/MPG.0000000000000192. [DOI] [PubMed] [Google Scholar]
- 34.Kaye RD, Towbin RB. Imaging and intervention in the gastrointestinal tract in children. Gastroenterol Clin North Am. 2002;31:897–923, viii. doi: 10.1016/s0889-8553(02)00024-9. [DOI] [PubMed] [Google Scholar]
- 35.Racadio JM, Agha AK, Johnson ND, Warner BW. Imaging and radiological interventional techniques for gastrointestinal bleeding in children. Semin Pediatr Surg. 1999;8:181–192. doi: 10.1016/s1055-8586(99)70025-9. [DOI] [PubMed] [Google Scholar]
- 36.Hongsakul K, Pakdeejit S, Tanutit P. Outcome and predictive factors of successful transarterial embolization for the treatment of acute gastrointestinal hemorrhage. Acta Radiol. 2014;55:186–194. doi: 10.1177/0284185113494985. [DOI] [PubMed] [Google Scholar]
- 37.Wei KL, Tung SY, Sheen CH, Chang TS, Lee IL, Wu CS. Effect of oral esomeprazole on recurrent bleeding after endoscopic treatment of bleeding peptic ulcers. J Gastroenterol Hepatol. 2007;22:43–46. doi: 10.1111/j.1440-1746.2006.04354.x. [DOI] [PubMed] [Google Scholar]
- 38.D'Amico G, Pietrosi G, Tarantino I, Pagliaro L. Emergency sclerotherapy versus vasoactive drugs for variceal bleeding in cirrhosis: a Cochrane meta-analysis. Gastroenterology. 2003;124:1277–1291. doi: 10.1016/s0016-5085(03)00269-5. [DOI] [PubMed] [Google Scholar]
- 39.Cardile S, Martinelli M, Barabino A, Gandullia P, Oliva S, Di Nardo G, Dall’Oglio L, Rea F, de’Angelis GL, Bizzarri B, et al. Italian survey on non-steroidal anti-inflammatory drugs and gastrointestinal bleeding in children. World J Gastroenterol. 2016;22:1877–1883. doi: 10.3748/wjg.v22.i5.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Burroughs AK. Somatostatin and octreotide for variceal bleeding. J Hepatol. 1991;13:1–4. doi: 10.1016/0168-8278(91)90854-5. [DOI] [PubMed] [Google Scholar]
- 41.Starship Children’s Health Clinical Guideline. Paediatric Gastroenterology. 2010. Available from: http://www.adhb.govt.nz/starshipclinicalguidelines/_Documents/GI bleeding.pdf. [Google Scholar]
- 42.Ioannou GN, Doust J, Rockey DC. Systematic review: terlipressin in acute oesophageal variceal haemorrhage. Aliment Pharmacol Ther. 2003;17:53–64. doi: 10.1046/j.1365-2036.2003.01356.x. [DOI] [PubMed] [Google Scholar]
- 43.Turnes J, Garcia-Pagan JC, Abraldes JG, Hernandez-Guerra M, Dell’Era A, Bosch J. Pharmacological reduction of portal pressure and long-term risk of first variceal bleeding in patients with cirrhosis. Am J Gastroenterol. 2006;101:506–512. doi: 10.1111/j.1572-0241.2006.00453.x. [DOI] [PubMed] [Google Scholar]
- 44.Bureau C, Péron JM, Alric L, Morales J, Sanchez J, Barange K, Payen JL, Vinel JP. “A La Carte” treatment of portal hypertension: Adapting medical therapy to hemodynamic response for the prevention of bleeding. Hepatology. 2002;36:1361–1366. doi: 10.1053/jhep.2002.36945. [DOI] [PubMed] [Google Scholar]
- 45.Thalheimer U, Bosch J, Burroughs AK. How to prevent varices from bleeding: shades of grey--the case for nonselective beta blockers. Gastroenterology. 2007;133:2029–2036. doi: 10.1053/j.gastro.2007.10.028. [DOI] [PubMed] [Google Scholar]
- 46.Villanueva C, Balanzó J, Novella MT, Soriano G, Sáinz S, Torras X, Cussó X, Guarner C, Vilardell F. Nadolol plus isosorbide mononitrate compared with sclerotherapy for the prevention of variceal rebleeding. N Engl J Med. 1996;334:1624–1629. doi: 10.1056/NEJM199606203342502. [DOI] [PubMed] [Google Scholar]
- 47.Erkek N, Senel S, Hizli S, Karacan CD. Terlipressin saved the life of a child with severe nonvariceal upper gastrointestinal bleeding. Am J Emerg Med. 2011;29:133.e5–133.e6. doi: 10.1016/j.ajem.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 48.Zaki SA. Terlipressin-induced hyponatremic seizure in a child. Indian J Pharmacol. 2013;45:403–404. doi: 10.4103/0253-7613.114995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Villanueva C, Miñana J, Ortiz J, Gallego A, Soriano G, Torras X, Sáinz S, Boadas J, Cussó X, Guarner C, et al. Endoscopic ligation compared with combined treatment with nadolol and isosorbide mononitrate to prevent recurrent variceal bleeding. N Engl J Med. 2001;345:647–655. doi: 10.1056/NEJMoa003223. [DOI] [PubMed] [Google Scholar]
- 50.El-Shabrawi MH, Kamal NM. Medical management of chronic liver diseases (CLD) in children (part II): focus on the complications of CLD, and CLD that require special considerations. Paediatr Drugs. 2011;13:371–383. doi: 10.2165/11591620-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 51.Collins R, Langman M. Treatment with histamine H2 antagonists in acute upper gastrointestinal hemorrhage. Implications of randomized trials. N Engl J Med. 1985;313:660–666. doi: 10.1056/NEJM198509123131104. [DOI] [PubMed] [Google Scholar]
- 52.de Franchis R. Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015;63:743–752. doi: 10.1016/j.jhep.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 53.Hwang JH, Fisher DA, Ben-Menachem T, Chandrasekhara V, Chathadi K, Decker GA, Early DS, Evans JA, Fanelli RD, Foley K, et al. The role of endoscopy in the management of acute non-variceal upper GI bleeding. Gastrointest Endosc. 2012;75:1132–1138. doi: 10.1016/j.gie.2012.02.033. [DOI] [PubMed] [Google Scholar]
- 54.Cappell MS. Therapeutic endoscopy for acute upper gastrointestinal bleeding. Nat Rev Gastroenterol Hepatol. 2010;7:214–229. doi: 10.1038/nrgastro.2010.24. [DOI] [PubMed] [Google Scholar]
- 55.Ünal F, Çakır M, Baran M, Duygulu Ş, Aydoğdu S. Application of endoscopic hemoclips for nonvariceal upper gastrointestinal bleeding in children. Turk J Gastroenterol. 2014;25:147–151. doi: 10.5152/tjg.2014.3419. [DOI] [PubMed] [Google Scholar]
- 56.Marmo R, Rotondano G, Piscopo R, Bianco MA, D’Angella R, Cipolletta L. Dual therapy versus monotherapy in the endoscopic treatment of high-risk bleeding ulcers: a meta-analysis of controlled trials. Am J Gastroenterol. 2007;102:279–289; quiz 469. doi: 10.1111/j.1572-0241.2006.01023.x. [DOI] [PubMed] [Google Scholar]
- 57.Laine L, McQuaid KR. Endoscopic therapy for bleeding ulcers: an evidence-based approach based on meta-analyses of randomized controlled trials. Clin Gastroenterol Hepatol. 2009;7:33–47; quiz 1-2. doi: 10.1016/j.cgh.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 58.Dai C, Liu WX, Jiang M, Sun MJ. Endoscopic variceal ligation compared with endoscopic injection sclerotherapy for treatment of esophageal variceal hemorrhage: a meta-analysis. World J Gastroenterol. 2015;21:2534–2541. doi: 10.3748/wjg.v21.i8.2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hwang JH, Shergill AK, Acosta RD, Chandrasekhara V, Chathadi KV, Decker GA, Early DS, Evans JA, Fanelli RD, Fisher DA, et al. The role of endoscopy in the management of variceal hemorrhage. Gastrointest Endosc. 2014;80:221–227. doi: 10.1016/j.gie.2013.07.023. [DOI] [PubMed] [Google Scholar]
- 60.Shneider BL, Bosch J, de Franchis R, Emre SH, Groszmann RJ, Ling SC, Lorenz JM, Squires RH, Superina RA, Thompson AE, et al. Portal hypertension in children: expert pediatric opinion on the report of the Baveno v Consensus Workshop on Methodology of Diagnosis and Therapy in Portal Hypertension. Pediatr Transplant. 2012;16:426–437. doi: 10.1111/j.1399-3046.2012.01652.x. [DOI] [PubMed] [Google Scholar]


