Figure 6. Hv1Sper and Hv1 form heterodimers with unique biophysical properties.
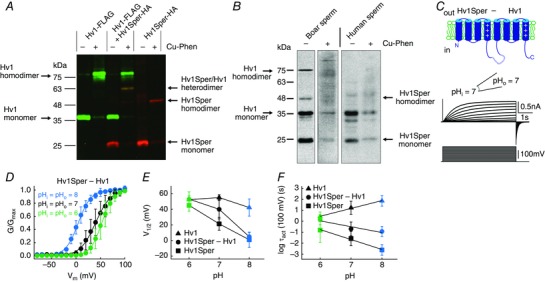
A, western blot of X. laevis oocytes expressing Hv1‐FLAG or Hv1Sper‐HA, or both, in the presence or absence of the oxidant Cu‐phenanthroline, probed with an anti‐FLAG (green) and anti‐HA (red) antibody. B, western blot of boar and human sperm incubated in the presence or absence of the cysteine cross‐linker Cu‐phenanthroline (Cu‐Phen), probed with a C‐terminal anti‐Hv1 antibody. C, inside‐out patch clamp recording from Xenopus oocytes expressing a tandem heterodimer consisting of Hv1Sper linked to Hv1 with a flexible linker, recorded at pHi = pHo = 7. Voltage steps of increasing amplitudes (–80 to +100 mV) elicited voltage‐dependent outward currents. D, GV relationships derived from tail currents at three different pH conditions (leaving ΔpH = 0), fitted with a single Boltzmann function. E, half‐maximal activation voltage (V 1/2) derived from the Boltzmann fits shown in (C) (Hv1 and Hv1Sper taken from Fig. 5 C). F, activation time constants of the Hv1Sper–Hv1 tandem heterodimer, as well as Hv1 and Hv1Sper (taken from Fig. 5 D). Error bars indicate the SD.
