Figure 4. Model showing the molecular mechanism by which alternative splicing of exon 29 regulates the voltage dependence of activation and open probability of CaV1.1.
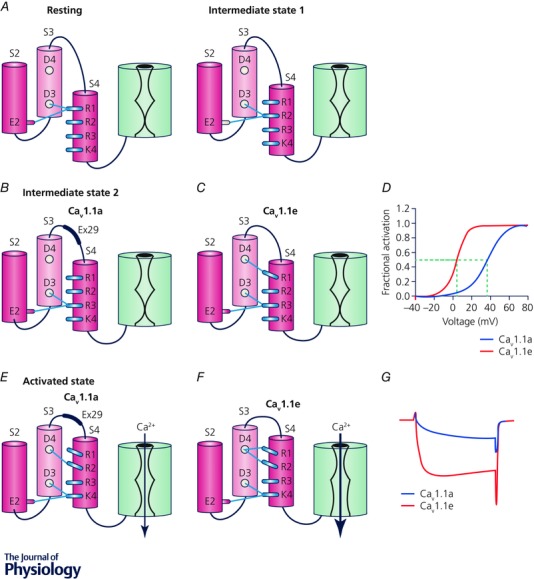
A, in the resting and intermediate state 1, R1 and R2, respectively, interact with two countercharges (glutamate E2 in IVS2 and aspartate D3 in IVS3) forming the charge transfer centre. Consequently there are no interactions of the IVS4 arginines with D4 of IVS3 and also no modulation by insertion/exclusion of exon 29 in the IVS3–S4 linker. B–D, in the intermediate state 2, R3 occupies the charge transfer centre and only in the absence of exon 29 (CaV1.1e) does R1 establish an interaction with D4 that facilitates the transition of the voltage sensor into the active state and therefore shifts the voltage dependence of CaV1.1e to the left. E and F, in the activated state, D4 of CaV1.1e forms several hydrogen bonds with both R1 and R2, thus stabilizing the activated state and increasing the open probability. B and E, inclusion of exon 29 in the IVS3–S4 linker of the adult splice variant CaV1.1a reduces the number and strength of the hydrogen bonds between D4 and R1 and R2. Consequently channel gating is severely perturbed and currents curtailed in the adult splice variant of the skeletal muscle calcium channel.
