Abstract
Key points
Common complications of pregnancy, such as chronic fetal hypoxia, trigger a fetal origin of cardiovascular dysfunction and programme cardiovascular disease in later life.
Sildenafil treatment protects placental perfusion and fetal growth, but whether the effects of sildenafil transcend the placenta to affect the fetus is unknown.
Using the chick embryo model, here we show that sildenafil treatment directly protects the fetal cardiovascular system in hypoxic development, and that the mechanisms of sildenafil protection include reduced oxidative stress and increased nitric oxide bioavailability;
Sildenafil does not protect against fetal growth restriction in the chick embryo, supporting the idea that the protective effect of sildenafil on fetal growth reported in mammalian studies, including humans, is secondary to improved placental perfusion.
Therefore, sildenafil may be a good candidate for human translational antioxidant therapy to protect the chronically hypoxic fetus in adverse pregnancy.
Abstract
There is a need for developing clinically translatable therapy for preventing fetal origins of cardiovascular disease in pregnancy complicated by chronic fetal hypoxia. Evidence shows that sildenafil protects placental perfusion and fetal growth. However, whether beneficial effects of sildenafil transcend onto the fetal heart and circulation in complicated development is unknown. We isolated the direct effects of sildenafil on the fetus using the chick embryo and hypothesised that sildenafil also protects fetal cardiovascular function in hypoxic development. Chick embryos (n = 11 per group) were incubated in normoxia or hypoxia (14% O2) from day 1 and treated with sildenafil (4 mg kg−1 day−1) from day 13 of the 21‐day incubation. Hypoxic incubation increased oxidative stress (4‐hydroxynonenal, 141.1 ± 17.6% of normoxic control), reduced superoxide dismutase (60.7 ± 6.3%), increased phosphodiesterase type 5 expression (167 ± 13.7%) and decreased nitric oxide bioavailability (54.7 ± 6.1%) in the fetal heart, and promoted peripheral endothelial dysfunction (70.9 ± 5.6% AUC of normoxic control; all P < 0.05). Sildenafil treatment after onset of chronic hypoxia prevented the increase in phosphodiesterase expression (72.5 ± 22.4%), protected against oxidative stress (94.7 ± 6.2%) and normalised nitric oxide bioavailability (115.6 ± 22.3%) in the fetal heart, and restored endothelial function in the peripheral circulation (89.8 ± 2.9%). Sildenafil protects the fetal heart and circulation directly in hypoxic development via mechanisms including decreased oxidative stress and enhanced nitric oxide bioavailability. Sildenafil may be a good translational candidate for human antioxidant therapy to prevent fetal origins of cardiovascular dysfunction in adverse pregnancy.
Keywords: Antioxidant, endothelial function, fetus, sildenafil
Key points
Common complications of pregnancy, such as chronic fetal hypoxia, trigger a fetal origin of cardiovascular dysfunction and programme cardiovascular disease in later life.
Sildenafil treatment protects placental perfusion and fetal growth, but whether the effects of sildenafil transcend the placenta to affect the fetus is unknown.
Using the chick embryo model, here we show that sildenafil treatment directly protects the fetal cardiovascular system in hypoxic development, and that the mechanisms of sildenafil protection include reduced oxidative stress and increased nitric oxide bioavailability;
Sildenafil does not protect against fetal growth restriction in the chick embryo, supporting the idea that the protective effect of sildenafil on fetal growth reported in mammalian studies, including humans, is secondary to improved placental perfusion.
Therefore, sildenafil may be a good candidate for human translational antioxidant therapy to protect the chronically hypoxic fetus in adverse pregnancy.
Abbreviations
- 3‐NT
3‐nitrotyrosine
- 4‐HNE
4‐hydroxynonenal
- ACh
acetylcholine
- COX
cyclooxygenase
- GMP
guanosine monophosphate
- GPx
glutathione peroxidase
- H
hypoxic
- HS
hypoxic sildenafil
- IUGR
intrauterine growth restriction
- N
normoxic
- NO
nitric oxide
- NOx
nitric oxide species
- NS
normoxic sildenafil
- PDE5
phosphodiesterase type 5
- PE
phenylephrine
- SNP
sodium nitroprusside
- SOD
superoxide dismutase
- ROS
reactive oxygen species
Introduction
It is widely accepted from data derived from humans and animal models that adverse conditions during pregnancy can trigger fetal growth restriction and an early origin of cardiovascular disease (Barker et al. 1993; Gluckman et al. 2008; Giussani & Davidge, 2013). Chronic fetal hypoxia is common during adverse pregnancy (Giussani, 2016) and independent studies have shown that chronic fetal hypoxia can trigger cardiovascular dysfunction in the offspring secondary to oxidative stress (Giussani et al. 2012; Patterson et al. 2012; Thompson & Al‐Hasan, 2012; Giussani & Davidge, 2013). For instance, hypoxic pregnancy in rats increased levels of oxidative stress in the fetal heart and vasculature, setting cardiac sympathetic dominance and endothelial dysfunction in the adult offspring; maternal treatment with the antioxidant vitamin C was protective (Giussani et al. 2012; Kane et al. 2013). Although such studies provided proof‐of‐principle to support the idea that maternal antioxidants protect against fetal origins of cardiovascular dysfunction in the chronically hypoxic fetus, only high doses of vitamin C incompatible with human treatment were effective. Further, in these studies maternal antioxidant therapy was administered from the onset of chronic fetal hypoxia, limiting their human translational capacity (Giussani et al. 2012; Kane et al. 2013). Clinically, diagnosis prior to treatment is necessary, therefore maternal antioxidant treatment following established chronic fetal hypoxia would provide a better translational study design.
One possible alternative candidate therapy is sildenafil, the selective inhibitor of cyclic guanosine monophosphate (GMP)‐specific phosphodiesterase type 5 (PDE5). Sildenafil has direct antioxidant properties (Koupparis et al. 2005) and by preventing the hydrolysis of cyclic GMP by PDE5, it additionally increases the bioavailability of cyclic GMP, a downstream secondary messenger of the potent vasodilator nitric oxide (NO, Francis & Corbin, 2003). Sildenafil treatment in human, ovine and murine pregnancy complicated by fetal growth restriction improved placental perfusion, increasing umbilical blood flow and protecting fetal growth (Satterfield et al. 2010; von Dadelszen et al. 2011; Stanley et al. 2012; Dilworth et al. 2013). Therefore, a large multicentre international scheme was recently launched to determine the efficacy of sildenafil as candidate human clinical intervention for fetal growth restriction (Ganzevoort et al. 2014). However, whether the positive effects of sildenafil transcend those on placental perfusion and fetal growth onto beneficial effects on the fetal cardiovascular system is completely unknown. Equally important, whether sildenafil has any potential adverse effects on fetal cardiovascular function in addition to effects on the maternal and/or placental physiology in healthy or complicated development is unclear.
Therefore, this study isolated the effects of sildenafil on the fetal cardiovascular system using the chick embryo, the only established animal model in which the direct effects on the fetal heart and circulation of potential therapy can be investigated, independent of effects on the mother and/or the placenta. The study tested the hypothesis that sildenafil treatment has direct beneficial effects on the fetal cardiovascular system in development complicated by chronic fetal hypoxia. In addition, we proposed that mechanisms of protection include reduced oxidative stress with enhanced NO bioavailability. The hypothesis was tested by investigating the effects of normoxic or hypoxic incubation of chick embryos with or without sildenafil treatment on fetal growth, fetal peripheral vascular reactivity and on molecular indices of oxidative stress, antioxidant capacity and NO bioavailability. Treatment of chick embryos with sildenafil started at day 13 of incubation, equivalent to ca 25 weeks of gestation in human pregnancy, a gestational age at which human fetal growth restriction can be reliably diagnosed.
Methods
Ethical approval
All procedures were performed under the UK Animals (Scientific Procedures) Act 1986 and were approved by the Ethical Review Committee of the University of Cambridge, as described in the Editorial by Grundy (2015).
Animals
Fertilised Bovans Brown eggs (Medeggs, Norfolk, UK) were weighed and incubated under normoxic (21% O2) or hypoxic (14 ± 0.5% O2) conditions (37.9°C, 45% humidity, 12:12 h light:dark cycle, automatic rotation every hour, Mod‐75A equipped with electronic servo‐controlled humidity system HS‐Auto‐3.5 l, Marsalles, Barcelona, Spain) from day 1. The levels of oxygen, humidity and temperature inside the incubators were continuously monitored (DD103 DrDAQ Oxygen Sensor, Pico Technology, St Neots, UK).
Dose of sildenafil
In clinical studies in which sildenafil has been administered to pregnant women, the dose varies between 0.86 and 3.43 mg kg−1 day−1 (Samangaya et al. 2009; von Dadelszen et al. 2011) assuming a 60 kg body weight at pre‐conception and a weight gain of 10 kg by 25 weeks of gestation (Bhattacharya et al. 2007; Fraser et al. 2010). The dose of chronic sildenafil treatment used in animal studies varies between 0.5 and 90 mg kg−1 day−1 (Refuerzo et al. 2006; Sanchez‐Aparicio et al. 2008). Notably, the metabolism of sildenafil also differs between species (Walker et al. 1999) and no pharmacokinetic study of sildenafil in the chicken has been previously reported. Collectively, from previous human and animal data available, a 4 mg kg−1 day−1 dose regimen was chosen for the present study as a dose that is clinically as well as scientifically relevant in humans. Therefore, chick embryos were treated with sildenafil (4 mg kg−1 day−1, Sildenafil citrate salt, Sigma‐Aldrich, UK) or vehicle (100 μl water) from day 13 to day 18 of incubation. Sildenafil was injected daily into the air cell onto the chorioallantoic membrane via a 1 mm hole in the eggshell of normoxic or hypoxic eggs. N = 11 eggs were used per group (normoxic control, hypoxic control, hypoxic sildenafil, normoxic sildenafil). The hole was covered with tape at all other times. All treatment procedures were performed under sterile conditions.
Haematocrit and growth analysis
On day 19 of the 21 day incubation period, embryos were killed by decapitation and immediately postmortem the weight of the embryo, yolk, extra‐embryonic membranes, chorioallantoic fluid and the shell was recorded and expressed as percentages of the egg weight on day 19 to determine how much resource was turned into fetal body mass within each egg. Blood was collected in micro‐haematocrit tubes (Vitrex, Modulohm, Denmark) directly from the heart and the haematocrit was determined in duplicate. Body length was measured by placing the ends of a digital caliper on top of the head and the base of the tail. The heart, brain, liver, lungs and the kidneys were dissected and weighed. A section of the third order femoral artery was dissected for vascular reactivity analysis. The heart was snap frozen in liquid nitrogen and stored at −80°C until molecular analysis.
Molecular studies in the chick embryo heart
Alterations in the pro‐oxidant indices 3‐nitrotyrosine (3‐NT) and 4‐hydroxynonenal (4‐HNE), in the antioxidant enzymes superoxide dismutase (SOD), catalase and glutathione peroxidase (GPx), and in the level of NO species (NOx) were determined in the chick embryo heart at day 19 of incubation. In addition, changes in the cardiac expression of PDE5 were determined to validate the effect of sildenafil treatment.
The expression of 3‐NT, 4‐HNE and SOD, the activity of catalase and the levels of NOx were determined by commercial assay kits according to the manufacturers’ instructions (3‐NT: ab116691, Abcam, Cambridge, UK; 4‐HNE: E12H0203, AMS biotechnology, Abington, UK; SOD: Sigma‐Aldrich, UK; Catalase: 707002 and NOx: 78001, Cayman Chemical Company, MI, USA).
The cardiac expression of GPx and PDE5 was determined by Western blot. Frozen chick hearts (25 mg) were powdered on dry ice and homogenised in 250 μl of ice‐cold lysis buffer (Hepes:50 mm, NaCl:150 mm, Triton‐X100:1%, Na3VO4:1 mm, NaF:30 mm, Na4P2O7:10 mm, EDTA:10 mm, protease inhibitor cocktail III (Calbiochem, Nottingham, UK)) in a microtube containing 1.4 mm ceramic beads (Lysing Matrix D, MP Biomedicals). The protein concentration of lysates was determined using the copper‐bicinchoninic assay (Smith et al. 1985). Protein samples were diluted with ×5 Laemmli's buffer (sodium dodecyl sulphate (SDS):2%, Tris‐HCl (pH 6.8):62.5 mm, glycerol:10%, dithiothreitol:100 mm, Bromophenol Blue) then standardised to a protein concentration of 1 mg ml−1 by further addition of ×1 Laemmli's buffer. Total protein (10 μg) from each sample (n = 6 per treatment group) was separated on SDS‐PAGE gel electrophoresis along with a pre‐stained molecular weight marker (PageRuler Plus Prestained Protein Ladder 10–250 kDa, Thermo Scientific, Waltham, USA), then transferred immediately to a polyvinylidene difluoride Immobilon‐P membrane (PVDF, Millipore, Billerica, MA, USA). The membrane was incubated in blocking buffer before incubation in primary antibodies (GPx, ab22604; PDE5, ab64179; Abcam) overnight at 4°C. Following primary antibody incubation the membranes were incubated with the secondary antibody (peroxidase‐AffiniPure donkey anti‐rabbit IgG (H+L), 1:10000 in PBS with 1% marvel and 0.1% Tween 20, Jackson ImmunoResearch Laboratories, PA, USA) and immunoreactivity was measured using West Pico chemiluminescent substrate (Thermo Scientific). The intensity of the bands were analysed with the AlphaEase imaging software (Alpha Innotech, San Leandro, CA, USA). Following the protein detection, the membrane was stained with 0.1% Coomassie R‐250 and the intensity of the Coomassie staining for each lane was analysed (Welinder & Ekblad, 2011). The expression level of the target protein in each sample was normalised to the Coomassie staining of the same sample for a loading control.
Functional peripheral vascular reactivity using in vitro wire myography
Constrictor and dilator function of the peripheral resistance vasculature was assessed using a microvascular myograph (Wire Myograph System 610M; DMT, Aarhus, Denmark) as previously described (Itani et al. 2016). Briefly, a third‐order femoral artery was dissected at day 19 of incubation and mounted in a chamber containing Kreb's buffer. Vascular constrictor capacity was assessed with increasing doses of K+ solutions (16.74–250 mm) and of phenylephrine (PE, 10−8–10−4 m). The response to K+ was normalised to the diameter of the vessel (mN mm−1 μm−1 1000−1). The response to phenylephrine was normalised to the constrictor response to 125 mm K+ achieved by the same vessel (% K+125 mm). Vasodilator responses to cumulative doses of sodium nitroprusside (SNP, 10−10–10−4 m) and of acetylcholine (ACh, 10−9–10−5 m) were assessed after pre‐constricting the vessel with a suboptimal dose of potassium. The partial contributions of endogenous NO‐dependent and NO‐independent mechanisms to the vasorelaxation were determined by repeating the ACh dose–response curve after incubating the vessel with l‐NAME (10−5 m, 10 min) and calculating the area under the curves (Herrera et al. 2010; Itani et al. 2016). The sensitivity (pD2) to ACh was defined as –log10 (EC50). LabChart was used for data acquisition and analysis of the in vitro wire myography data (LabChart 6.0, Powerlab 8/30; AD Instruments, Chalgrove, UK).
Statistical analysis
All data are expressed as means ± SEM Data were checked for Gaussian distribution using the D'Agostino‐Pearson normality test. Statistical comparisons were made using two‐way ANOVA, with the Bonferroni post hoc test where a significant interaction was detected. For all comparisons, statistical significance was accepted when P < 0.05. (Graphpad prism version 5.00, Graphpad Software, Inc. San Diego, CA, USA).
Results
Haematocrit and fetal growth
Incubation under hypoxic conditions from day 1 significantly increased haematocrit in the chick embryo by day 19 (Fig. 1 A). Exposure to hypoxia throughout development reduced the body weight (Fig. 1 B) which persisted when the body weight was normalised to the egg weight at the start of incubation (N: 41.3 ± 1.0, H: 29.0 ± 1.3*, HS: 32.0 ± 2.2*, NS: 43.9 ± 1.3 g g−1, P < 0.05; *effect of hypoxia). Hypoxia affected the body weight more severely than the body length (N: 68.1 ± 1.7, H: 63.2 ± 1.0*, HS: 65.9 ± 0.9, NS: 70.8 ± 0.6 mm, P < 0.05; * versus N) of the embryo. Consequently, hypoxic embryos had a lower BMI (N: 5.3 ± 0.2, H: 4.6 ± 0.1*, HS: 4.5 ± 0.2*, NS: 5.1 ± 0.1 kg m−2, P < 0.05; *effect of hypoxia). The brain weight was reduced in the hypoxic embryo (N: 0.84 ± 0.01, H: 0.73 ± 0.02*, HS: 0.74 ± 0.01*, NS: 0.84 ± 0.01 g, P < 0.05; *effect of hypoxia), however, this was not proportional to the reduction in their body size and thus, the relative brain weight was increased (Fig. 1 C). In addition, calculation of resource partitioning revealed less resource attributed to embryonic mass in hypoxic incubations (Fig. 1 D). Collectively, the data show that those embryos exposed to chronic hypoxia were thin for their length and had relative brain sparing. Sildenafil treatment from day 13 of incubation had no effect on changes in fetal growth or brain sparing during either hypoxic or normoxic incubation (Fig. 1).
Figure 1. Haematocrit and fetal biometry.
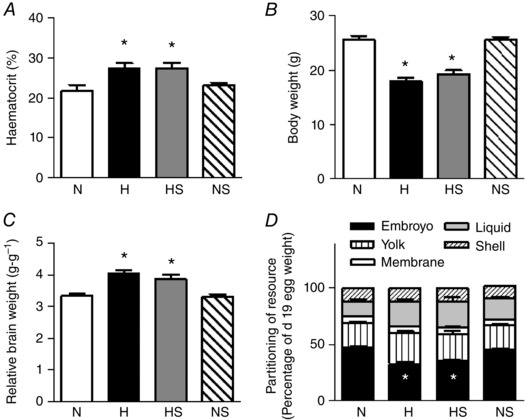
Values are means ± SEM at day 19 of haematocrit (A), embryo weight (B), brain weight relative to body weight (C), and resource partitioning (D) of chick embryos incubated in either normoxia (N, n = 11), hypoxia (H, n = 10), hypoxia with sildenafil (HS, n = 10) or normoxia with sildenafil (NS, n = 11). Significant (P < 0.05) differences are: *effect of hypoxia (N vs. H and NS vs. HS); two‐way ANOVA. There was no interaction found between the effect of hypoxia and of sildenafil treatment.
Exposure to chronic hypoxia from day 1 significantly reduced the weight of the heart, lungs, liver and kidneys by day 19 in the chick embryo, and this was proportional to the reduction in body size (Table 1). Sildenafil treatment in normoxic and hypoxic embryos did not affect the absolute or relative weight of the lungs, liver or kidneys. However, both absolute and relative heart weights were significantly reduced in normoxic embryos treated with sildenafil, while the relative heart weight was also reduced in hypoxic embryos treated with sildenafil (Table 1).
Table 1.
Organ weight of chick embryos at day 19 of incubation
| N | H | HS | NS | Overall effect of | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | Hypoxia | Sildenafil | |
| Heart | 0.2 | 0.01 | 0.14 | 0.01* | 0.13 | 0.01* | 0.17 | 0† | P < 0.0001 | — |
| Lung | 0.24 | 0.01 | 0.14 | 0.02* | 0.16 | 0.01* | 0.23 | 0.01 | P < 0.0001 | — |
| Liver | 0.57 | 0.02 | 0.39 | 0.02* | 0.42 | 0.01* | 0.53 | 0.02 | P < 0.0001 | — |
| Kidney | 0.23 | 0.01 | 0.16 | 0.02* | 0.18 | 0.01* | 0.23 | 0.01 | P < 0.0001 | — |
| Heart BW−1 | 0.77 | 0.02 | 0.8 | 0.04 | 0.66 | 0.03† | 0.67 | 0.02† | — | P < 0.0001 |
| Lung BW−1 | 0.92 | 0.03 | 0.84 | 0.11 | 0.85 | 0.06 | 0.91 | 0.03 | — | — |
| Liver BW−1 | 2.16 | 0.08 | 2.15 | 0.1 | 2.21 | 0.08 | 2.07 | 0.04 | — | — |
| Kidney BW−1 | 0.88 | 0.03 | 0.89 | 0.07 | 0.91 | 0.05 | 0.89 | 0.03 | — | — |
Values are means and SEM at day 19 of absolute (in grams) and relative organ weights (to body weight, BW, in g g−1) of chick embryos incubated in either N (n = 11), H (n = 10), HS (n = 10) or NS (n = 11). Significant (P < 0.05) differences are: *effect of hypoxia (N vs. H and NS vs. HS): †effect of sildenafil (N vs. NS and H vs. HS); two‐way ANOVA. There was no interaction found between the effect of hypoxia and of sildenafil treatment.
Molecular studies in the chick embryo heart
In the heart of chick embryos exposed to chronic hypoxia, the protein expression of 3‐NT and 4‐HNE was significantly elevated (Fig. 2 A and B). In addition, the expression of SOD and the activity of catalase were both decreased in the hypoxic heart (Fig. 3 A and B). Hypoxic incubation had no effect on the cardiac expression of GPx (Fig. 3 C) but the total cardiac NOx concentration was reduced (Fig. 3 D). Sildenafil treatment prevented the increase in 4‐HNE but not 3‐NT in the hypoxic embryo heart. Cardiac NOx levels were restored by sildenafil treatment in the hypoxic embryo. However, the treatment significantly reduced the levels of cardiac NOx in normoxic embryos. Sildenafil treatment had no significant effect on the expression of SOD or the activity of catalase, but the protein expression of GPx in the heart of hypoxic embryos was significantly elevated (Figs 2 and 3).
Figure 2. Pro‐oxidant mechanisms.
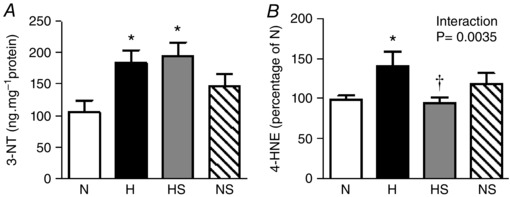
Values are means ± SEM at day 19 of the expression of 3‐NT (A), and 4‐HNE (B), in the heart of chick embryos incubated in either N, H, HS or NS (n = 8, 8, 9, 9, respectively). Significant (P < 0.05) differences are: *effect of hypoxia (N vs. H and NS vs. HS): †effect of sildenafil (N vs. NS and H vs. HS); two‐way ANOVA with no interaction (A) or with interaction and Bonferroni post hoc test (B).
Figure 3. Anti‐oxidant mechanisms and NO bioavailability.
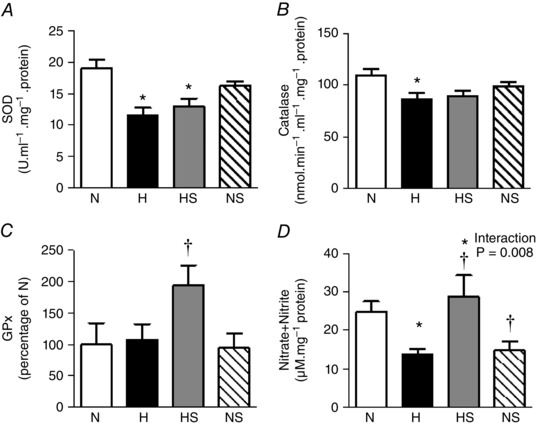
Values are means ± SEM at day 19 of the expression of SOD (A), the activity of catalase (B), the expression of GPx (C), and the concentration of NOx (D) in the heart of chick embryos incubated in either N, H, HS or NS (n = 9, 9, 8, 8, respectively, for A and B; n = 6 for all groups for C; n = 8, 8, 9, 9, respectively, for D). Significant (P < 0.05) differences are: *effect of hypoxia (N vs. H and NS vs. HS); †effect of sildenafil (N vs. NS and H vs. HS); two‐way ANOVA with no interaction (A, B and C) or with interaction and Bonferroni post hoc test (D).
Compared to normoxic embryos, the protein expression of PDE5 in the heart was significantly enhanced in hypoxic embryos treated with vehicle. Sildenafil treatment of hypoxic embryos normalised the protein expression of cardiac PDE5 and sildenafil treatment of normoxic embryos showed no effect on the protein expression of cardiac PDE5 (Fig. 4).
Figure 4. Cardiac PDE5 expression.
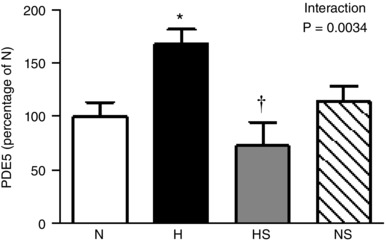
Values are means ± SEM at day 19 for the expression of PDE5 protein in the heart of chick embryos incubated in either N, H, HS or NS. n = 6 for all groups. Significant (P < 0.05) differences are: *effect of hypoxia (N vs. H): †effect of sildenafil (H vs. HS); two‐way ANOVA with interaction and Bonferroni post hoc test.
Functional peripheral vascular reactivity using in vitro wire myography
The femoral arterial segments displayed a dose‐dependent relaxation in response to SNP and ACh (Figs 5 and 6). The femoral arterial vascular response to SNP was not significantly affected by hypoxic incubation or sildenafil treatment (Fig. 6 A). In contrast, incubation under hypoxic conditions shifted the ACh relaxation curve to the right, with vessels requiring higher concentrations of ACh to achieve similar relaxation (Figs 5 and 6 B). Consequently, compared to normoxic embryos, the sensitivity (pD2) of the vessels to ACh was significantly reduced in hypoxic embryos. In addition the total relaxant capacity to ACh, measured as area under the curve (AUC), was significantly reduced in the hypoxic embryo (Fig 6 C). The ACh relaxant curve was repeated in the presence of a NO synthase blocker l‐NAME to determine the partial contributions of NO‐dependent and NO‐independent components of the vasodilatation, which revealed that the deficit in the total femoral vascular relaxation in response to ACh in hypoxic embryos was primarily due to NO‐independent mechanisms. Sildenafil treatment in hypoxic embryos rescued the vasodilatation both in terms of sensitivity and total relaxation by significantly enhancing the NO‐dependent component of the vasodilatation (Fig 6 B and C). Sildenafil treatment in normoxic embryos did not affect femoral vascular dilator function.
Figure 5. Representative recording of the acetylcholine dose–response curves.
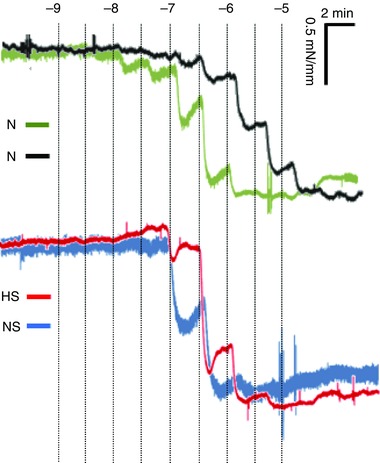
Example recordings of a femoral arterial segment of 2 mm that was exposed to cumulative doses of acetylcholine (ACh) isolated from chick embryos incubated in either N, H, HS or NS. The traces are shown as time (minutes, horizontal axis) vs. vascular wall tension (mN mm−1, vertical axis). The ACh doses were given at 2 min intervals. Concentration of ACh are shown as –log10 m.
Figure 6. Peripheral vasodilator function.
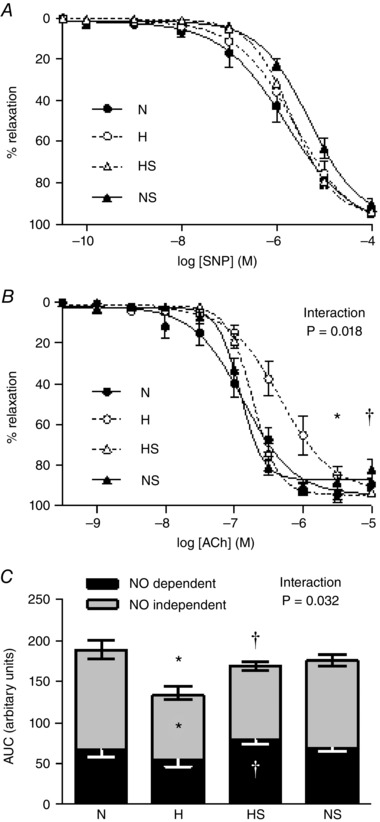
Values are means ± SEM for relaxant responses to SNP (A), and to ACh (B), and vasodilatation to ACh expressed as area under the curve before and after l‐NAME treatment (AUC, C) for femoral arterial segments isolated from chick embryos incubated in either N, H, HS and NS (n = 10 for all groups). In C, the AUC represents ACh‐induced relaxation (complete bar with positive SEM), for ACh‐induced relaxation following treatment with l‐NAME (NO‐independent component, grey bar with negative SEM), and for the remaining AUC after ACh with l‐NAME (NO‐dependent component, black bar with negative white SEM). Significant (P < 0.05) differences are: *effect of hypoxia (N vs. H): †effect of sildenafil (H vs. HS) for pD2 (B) and AUC (C); two‐way ANOVA with interaction and Bonferroni post hoc test.
Relative to normoxic embryos, those incubated under hypoxic conditions displayed a significantly enhanced maximal constrictor response to potassium (N: 2.4 ± 0.2, H: 4.4 ± 0.9*, HS: 4.4 ± 0.5*, NS: 2.5 ± 0.3 mN mm−1 μm−1 1000−1) but constrictor responses to phenylephrine were not affected (N:116 ± 4, H:118 ± 4, HS:125 ± 10, NS:124 ± 10% K+ 125 mm). Sildenafil treatment had no effect on femoral constrictor function.
Discussion
The data in this study show that hypoxic incubation of the chick embryo led to an increase in fetal haematocrit and promoted asymmetric fetal growth restriction by the end of the incubation period. Hypoxic incubation increased levels of oxidative stress in the fetal heart, reduced cardiac antioxidant defences, increased cardiac PDE5 expression, decreased cardiac NO bioavailability and promoted endothelial dysfunction in peripheral resistance vessels. Treatment of the chronically hypoxic chick embryo with sildenafil long after the onset of chronic hypoxia prevented the increase in cardiac PDE5 expression, protected against cardiac oxidative stress, normalised cardiac NO bioavailability and restored peripheral endothelial function. Therefore, the data support the hypothesis tested that sildenafil has direct beneficial effects on the fetal cardiovascular system in development complicated by chronic fetal hypoxia.
In addition to its obvious advantages of higher throughput and lower cost over other animal models, the chick embryo is the only established animal model that permits isolation of the direct effects on the fetus of developmental hypoxia, oxidative stress and/or treatment independent of additional effects of the experimental design on maternal nutrition or changes in the placental and/or maternal physiology. The ontogeny of cardiac development in the chicken is much more comparable to the human than the rat or the mouse (Marcela et al. 2012). Further, mechanisms underlying the control of cardiovascular function in the chick embryo and the human fetus show many similarities (Crossley & Altimiras, 2000; Mulder et al. 2000; Ruijtenbeek et al. 2002; Giussani, 2016). Consequently, the chick embryo model has been used by independent groups to isolate the effects on fetal growth and on fetal cardiovascular function of development complicated by chronic fetal hypoxia (Ruijtenbeek et al., 2000; Dzialowski et al. 2002; Rouwet et al. 2002; Sharma et al. 2006; Salinas et al. 2010; Giussani, 2016; Itani et al. 2016).
Accumulating evidence shows that PDE5 is involved in many cardiac disease states where perturbations in NO signalling are implicated (Kass et al. 2007). The expression of PDE5 is upregulated in the left ventricle in patients with end‐stage ischaemic or dilated cardiomyopathy (Pokreisz et al. 2009) and in hypertrophied hearts (Nagendran et al. 2007). Importantly, the increase in myocardial PDE5 in the failing heart is associated with increased levels of cardiac 3‐NT and 4‐HNE (Lu et al. 2010). Chronic hypoxia also stimulates a number of pro‐oxidant pathways such as xanthine‐oxidase (Kane et al. 2014), as well as consuming a number of antioxidant defences (Maiti et al. 2006; Giussani & Davidge, 2013). In the present study, treatment with sildenafil prevented the chronic hypoxia‐induced increase in PDE5 and in 4‐HNE, it increased glutathione and restored NO bioavailability in the chick embryo heart. 4‐HNE is a major product of lipid peroxidation and it is formed via several ROS‐dependent pathways (Spickett, 2013). GPx is an established antioxidant enzyme (Masella et al. 2005). Therefore, the restored levels of NOx and 4‐HNE coupled with the enhanced levels of GPx in the heart of sildenafil‐treated hypoxic chick embryos support direct antioxidant mechanisms of sildenafil on the fetal cardiovascular system.
Additional data presented in this study show that exposure to chronic hypoxia during development leads to impaired dilatation in the peripheral vasculature, predominantly through NO‐independent mechanisms. The binding of ACh to its trans‐membrane receptors on the endothelial cell increases the level of intracellular calcium (Ca2+), releasing arachidonic acid within the cell that is, in turn, converted into prostaglandins by cyclooxygenases COX1 and COX2 (Bachschmid et al. 2005). During chronic hypoxia, arachidonic acid is preferentially converted into constrictor prostaglandins, such as TXA2 and PGF2α via enhanced COX2 activity (Fike et al. 2005; Wong et al. 2009). It is well known that hypoxia at the tissue level leads to an increased generation of ROS, particularly the superoxide anion (•O2 −) at the mitochondria (Chandel et al. 1998; Becker et al. 1999). •O2 − readily combines with NO to limit its bioavailability (Kissner et al. 1997; Thakor et al. 2010 b). Therefore, chronic hypoxia shifts the cardiovascular phenotype into one of oxidative stress as well as switching the metabolism of arachidonic acid towards constrictor pathways (Fike et al. 2005; Delannoy et al. 2010; Giussani, 2016). In the present study, treatment with sildenafil of hypoxic chick embryos restored the endothelium‐dependent relaxation of the femoral artery by enhancing NO‐dependent mechanisms. This agrees with the principal dilator mechanism of action of sildenafil, enhancing downstream signalling of NO. The ratio of •O2 −:NO yields a vascular oxidant tone and we have shown that this is functional in fetal life and that it can be manipulated in favour of dilatation (Thakor et al. 2010 a, b ; Giussani et al. 2012; Kane et al. 2014). Mechanisms in addition to antioxidant properties underlying the beneficial effects of sildenafil in the chronically hypoxic fetus therefore include inhibition of the degradation of cyclic GMP by PDE5, enhancing the action of NO, thereby normalising the vascular oxidant tone and endothelial function.
Other data in the present study show that the femoral maximal contractile response to potassium was significantly enhanced in the chick embryo exposed to chronic hypoxia. The hypoxia‐induced increase in the femoral contractile capacity in the chronically hypoxic chick embryo may be a direct effect of hypoxia and/or secondary to the known effects of chronic hypoxia in promoting sympathetic hyperinnervation (Ruijtenbeek et al. 2000). The latter has also been associated with proliferation and differentiation of vascular smooth muscle cells (le Noble et al. 2000; Rouwet et al. 2002). In the present study, sildenafil treatment did not diminish the magnitude of the femoral constrictor responses to either phenylephrine or to potassium, further supporting that the mechanism of action mediating the improved peripheral vasodilatation is via enhancing NO‐dependent actions rather than by depressing constrictor mechanisms.
In the present study, treatment with sildenafil of hypoxic chick embryos did not improve the fetal growth restriction but brain sparing in growth‐restricted fetuses was preserved. Alterations in the ratio of •O2 −:NO promoting a vascular oxidant tone may have a significant effect on circulations which are particularly sensitive to NO, such as the placental and umbilical vascular bed (Derks et al. 2010; Thakor et al. 2010 a). In support, a number of studies in humans and mammalian animal models have reported possible protection by sildenafil against fetal growth restriction secondary to improved placental perfusion in complicated pregnancy (Refuerzo et al. 2006; Sanchez‐Aparicio et al. 2008; Satterfield et al. 2010; von Dadelszen et al. 2011; Herraiz et al. 2012; Stanley et al. 2012; Dilworth et al. 2013). In the present study, sildenafil did not prevent growth restriction in the chronically hypoxic chick embryo, supporting a protective effect of sildenafil on fetal growth in mammalian species by improving placental perfusion. Alternatively, it could be argued that sildenafil treatment in this model of hypoxic development started too late following the onset of chronic fetal hypoxia to prevent fetal growth restriction.
In conclusion, we have intertwined the use of the chick embryo with hypoxic incubation to provide the first evidence that sildenafil has direct protective effects on the fetal heart and vasculature, independent of the presence of a placenta. The mechanisms underlying the protection conveyed by sildenafil on the fetal cardiovascular system include inhibition of PDE5, increased antioxidant defences, diminished oxidative stress, increased NO bioavailability and diminished NO‐dependent endothelial dysfunction. The protective effects of sildenafil on the cardiovascular system of the chronically hypoxic fetus were evident even when sildenafil therapy was started long after the onset of chronic hypoxia. The lack of a protective effect of sildenafil treatment on fetal growth in the chick embryo during hypoxic development supports beneficial effects of sildenafil on growth in intrauterine growth restriction (IUGR) fetuses of mammalian species, including humans, to be at the level of the placenta. Future research will therefore need to consider direct and indirect effects of sildenafil at the maternal, placental and fetal levels. However, sildenafil is a plausible candidate for human clinical translational therapy to rescue adverse effects of pregnancy complicated by developmental hypoxia.
Additional information
Competing interests
None declared.
Author contributions
All experiments were performed at The University of Cambridge. Conception and design of experiments and analysis of data: N.I., K.L.S., C.B. and D.A.G. Editing of the article: N.I., D.A.G. All authors approve the final version of the manuscript and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
This work was supported by the British Heart Foundation. Dino Giussani is the Professor of Cardiovascular Developmental Physiology and Medicine at the Department of Physiology Development and Neuroscience at the University of Cambridge, Professorial Fellow and Director of Studies in Medicine at Gonville and Caius College, a Lister Institute Fellow and a Royal Society Wolfson Research Merit Award Holder.
Translational perspective
Accumulating data derived from humans and animal models of complicated pregnancy support potential beneficial effects of sildenafil in improving placental perfusion and protecting fetal growth. These findings have served as the basis for launching the STRIDER clinical trials, a large multi‐centre international scheme to determine the efficacy of sildenafil as candidate clinical interventional therapy to improve fetal growth restriction. However, whether the positive effects of sildenafil transcend those on placental perfusion and fetal growth onto beneficial effects on the fetal cardiovascular system was unknown. Equally important, whether sildenafil has any potential adverse effects on fetal cardiovascular function in healthy or complicated development was unclear. Here, we show that sildenafil has direct protective effects on the developing cardiovascular system of the chronically hypoxic fetus. Further, these protective effects are evident when sildenafil therapy is started long after the onset of chronic fetal hypoxia. This is useful from a human clinical perspective, as therapy can only be administered once fetal growth restriction as a result of chronic fetal hypoxia is diagnosed around 25 weeks of gestation. Therefore, sildenafil may be a good candidate for human translational antioxidant therapy to protect the chronically hypoxic fetus in adverse pregnancy.
References
- Bachschmid M, Schildknecht S & Ullrich V (2005). Redox regulation of vascular prostanoid synthesis by the nitric oxide‐superoxide system. Biochem Biophys Res Commun 338, 536–542. [DOI] [PubMed] [Google Scholar]
- Barker DJ, Osmond C, Simmonds SJ & Wield GA (1993). The relation of small head circumference and thinness at birth to death from cardiovascular disease in adult life. BMJ 306, 422–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker LB, vanden Hoek TL, Shao ZH, Li CQ & Schumacker PT (1999). Generation of superoxide in cardiomyocytes during ischemia before reperfusion. Am J Physiol Heart Circ Physiol 277, H2240–H2246. [DOI] [PubMed] [Google Scholar]
- Bhattacharya S, Campbell DM, Liston WA & Bhattacharya S (2007). Effect of body mass index on pregnancy outcomes in nulliparous women delivering singleton babies. BMC Public Health 7, 168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandel NS, Maltepe E, Goldwasser E, Mathieu CE, Simon MC & Schumacker PT (1998). Mitochondrial reactive oxygen species trigger hypoxia‐induced transcription. Proc Natl Acad Sci USA 95, 11715–11720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossley D & Altimiras J (2000). Ontogeny of cholinergic and adrenergic cardiovascular regulation in the domestic chicken (Gallus gallus). Am J Physiol Regul Integr Comp Physiol 279, R1091–R1098. [DOI] [PubMed] [Google Scholar]
- Delannoy E, Courtois A, Freund‐Michel V, Leblais V, Marthan R & Muller B (2010). Hypoxia‐induced hyperreactivity of pulmonary arteries: role of cyclooxygenase‐2, isoprostanes, and thromboxane receptors. Cardiovasc Res 85, 582–592. [DOI] [PubMed] [Google Scholar]
- Derks JB, Oudijk MA, Torrance HL, Rademaker CM, Benders MJ, Rosen KG, Cindrova‐Davies T, Thakor AS, Visser GH, Burton GJ, van Bel F & Giussani DA (2010). Allopurinol reduces oxidative stress in the ovine fetal cardiovascular system after repeated episodes of ischemia‐reperfusion. Pediatr Res 68, 374–380. [DOI] [PubMed] [Google Scholar]
- Dilworth MR, Andersson I, Renshall LJ, Cowley E, Baker P, Greenwood S, Sibley CP & Wareing M (2013). Sildenafil citrate increases fetal weight in a mouse model of fetal growth restriction with a normal vascular phenotype. PLoS One 8, e77748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzialowski EM, von Plettenberg D, Elmonoufy NA & Burggren WW (2002). Chronic hypoxia alters the physiological and morphological trajectories of developing chicken embryos. Comp Biochem Physiol A Mol Integr Physiol 131, 713–724. [DOI] [PubMed] [Google Scholar]
- Fike CD, Kaplowitz MR, Zhang Y & Pfister SL (2005). Cyclooxygenase‐2 and an early stage of chronic hypoxia‐induced pulmonary hypertension in newborn pigs. J Appl Physiol (1985) 98, 1111–1118; discussion 1091. [DOI] [PubMed] [Google Scholar]
- Francis SH & Corbin JD (2003). Molecular mechanisms and pharmacokinetics of phosphodiesterase‐5 antagonists. Curr Urol Rep 4, 457–465. [DOI] [PubMed] [Google Scholar]
- Fraser A, Tilling K, Macdonald‐Wallis C, Sattar N, Brion MJ, Benfield L, Ness A, Deanfield J, Hingorani A, Nelson SM, Smith GD & Lawlor DA (2010). Association of maternal weight gain in pregnancy with offspring obesity and metabolic and vascular traits in childhood. Circulation 121, 2557–2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganzevoort W, Alfirevic Z, von Dadelszen P, Kenny L, Papageorghiou A, van Wassenaer‐Leemhuis A, Gluud C, Mol BW & Baker PN (2014). STRIDER: Sildenafil Therapy In Dismal prognosis Early‐onset intrauterine growth Restriction – a protocol for a systematic review with individual participant data and aggregate data meta‐analysis and trial sequential analysis. Syst Rev 3, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giussani DA (2016). The fetal brain sparing response to hypoxia: physiological mechanisms. J Physiol 594, 1215–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giussani DA, Camm EJ, Niu Y, Richter HG, Blanco CE, Gottschalk R, Blake EZ, Horder KA, Thakor AS, Hansell JA, Kane AD, Wooding FB, Cross CM & Herrera EA (2012). Developmental programming of cardiovascular dysfunction by prenatal hypoxia and oxidative stress. PLoS One 7, e31017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giussani DA & Davidge ST (2013). Developmental programming of cardiovascular disease by prenatal hypoxia. J Dev Orig Health Dis 4, 328–337. [DOI] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA, Cooper C & Thornburg KL (2008). Effect of in utero and early‐life conditions on adult health and disease. N Engl J Med 359, 61–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy D (2015). Principles and standards for reporting animal experiments in The Journal of Physiology and Experimental Physiology . J Physiol 593, 2547–2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herraiz S, Pellicer B, Serra V, Cauli O, Cortijo J, Felipo V & Pellicer A (2012). Sildenafil citrate improves perinatal outcome in fetuses from pre‐eclamptic rats. BJOG 119, 1394–1402. [DOI] [PubMed] [Google Scholar]
- Herrera EA, Verkerk MM, Derks JB & Giussani DA (2010). Antioxidant treatment alters peripheral vascular dysfunction induced by postnatal glucocorticoid therapy in rats. PLoS One 5, e9250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itani N, Skeffington KL, Beck C, Niu Y & Giussani DA (2016). Melatonin rescues cardiovascular dysfunction during hypoxic development in the chick embryo. J Pineal Res 60, 16–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane AD, Hansell JA, Herrera EA, Allison BJ, Niu Y, Brain KL, Kaandorp JJ, Derks JB & Giussani DA (2014). Xanthine oxidase and the fetal cardiovascular defence to hypoxia in late gestation ovine pregnancy. J Physiol 592, 475–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane AD, Herrera EA, Camm EJ & Giussani DA (2013). Vitamin C prevents intrauterine programming of in vivo cardiovascular dysfunction in the rat. Circ J 77, 2604–2611. [DOI] [PubMed] [Google Scholar]
- Kass DA, Takimoto E, Nagayama T & Champion HC (2007). Phosphodiesterase regulation of nitric oxide signalling. Cardiovasc Res 75, 303–314. [DOI] [PubMed] [Google Scholar]
- Kissner R, Nauser T, Bugnon P, Lye PG & Koppenol WH (1997). Formation and properties of peroxynitrite as studied by laser flash photolysis, high‐pressure stopped‐flow technique, and pulse radiolysis. Chem Res Toxicol 10, 1285–1292. [DOI] [PubMed] [Google Scholar]
- Koupparis AJ, Jeremy JY, Muzaffar S, Persad R & Shukla N (2005). Sildenafil inhibits the formation of superoxide and the expression of gp47 NAD[P]H oxidase induced by the thromboxane A2 mimetic, U46619, in corpus cavernosal smooth muscle cells. BJU Int 96, 423–427. [DOI] [PubMed] [Google Scholar]
- le Noble FA, Ruijtenbeek K, Gommers S, de Mey JG & Blanco CE (2000). Contractile and relaxing reactivity in carotid and femoral arteries of chicken embryos. Am J Physiol Heart Circ Physiol 278, H1261–H1268. [DOI] [PubMed] [Google Scholar]
- Lu Z, Xu X, Hu X, Lee S, Traverse JH, Zhu G, Fassett J, Tao Y, Zhang P, dos Remedios C, Pritzker M, Hall JL, Garry DJ & Chen Y (2010). Oxidative stress regulates left ventricular PDE5 expression in the failing heart. Circulation 121, 1474–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiti P, Singh SB, Sharma AK, Muthuraju S, Banerjee PK & Ilavazhagan G (2006). Hypobaric hypoxia induces oxidative stress in rat brain. Neurochem Int 49, 709–716. [DOI] [PubMed] [Google Scholar]
- Marcela SG, Cristina RM, Angel PG, Manuel AM, Sofia DC, Patricia de LR, Bladimir RR & Concepcion SG (2012). Chronological and morphological study of heart development in the rat. Anat Rec (Hoboken) 295, 1267–1290. [DOI] [PubMed] [Google Scholar]
- Masella R, Di Benedetto R, Vari R, Filesi C & Giovannini C (2005). Novel mechanisms of natural antioxidant compounds in biological systems: involvement of glutathione and glutathione‐related enzymes. J Nutr Biochem 16, 577–586. [DOI] [PubMed] [Google Scholar]
- Mulder AL, Golde JM, Goor AA, Giussani DA & Blanco CE (2000). Developmental changes in plasma catecholamine concentrations during normoxia and acute hypoxia in the chick embryo. J Physiol 527, 593–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagendran J, Archer SL, Soliman D, Gurtu V, Moudgil R, Haromy A, St Aubin C, Webster L, Rebeyka IM, Ross DB, Light PE, Dyck JR & Michelakis ED (2007). Phosphodiesterase type 5 is highly expressed in the hypertrophied human right ventricle, and acute inhibition of phosphodiesterase type 5 improves contractility. Circulation 116, 238–248. [DOI] [PubMed] [Google Scholar]
- Patterson AJ, Xiao D, Xiong F, Dixon B & Zhang L (2012). Hypoxia‐derived oxidative stress mediates epigenetic repression of PKCepsilon gene in foetal rat hearts. Cardiovasc Res 93, 302–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pokreisz P, Vandenwijngaert S, Bito V, Van den Bergh A, Lenaerts I, Busch C, Marsboom G, Gheysens O, Vermeersch P, Biesmans L, Liu X, Gillijns H, Pellens M, Van Lommel A, Buys E, Schoonjans L, Vanhaecke J, Verbeken E, Sipido K, Herijgers P, Bloch KD & Janssens SP (2009). Ventricular phosphodiesterase‐5 expression is increased in patients with advanced heart failure and contributes to adverse ventricular remodeling after myocardial infarction in mice. Circulation 119, 408–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Refuerzo JS, Sokol RJ, Aranda JV, Hallak M, Hotra JW, Kruger M & Sorokin Y (2006). Sildenafil citrate and fetal outcome in pregnant rats. Fetal Diagn Ther 21, 259–263. [DOI] [PubMed] [Google Scholar]
- Rouwet EV, Tintu AN, Schellings MW, van Bilsen M, Lutgens E, Hofstra L, Slaaf DW, Ramsay G & Le Noble FA (2002). Hypoxia induces aortic hypertrophic growth, left ventricular dysfunction, and sympathetic hyperinnervation of peripheral arteries in the chick embryo. Circulation 105, 2791–2796. [DOI] [PubMed] [Google Scholar]
- Ruijtenbeek K, De Mey JG & Blanco CE (2002). The chicken embryo in developmental physiology of the cardiovascular system: a traditional model with new possibilities. Am J Physiol Regul Integr Comp Physiol 283, R549–R550; author reply R550–R541. [DOI] [PubMed] [Google Scholar]
- Ruijtenbeek K, le Noble FA, Janssen GM, Kessels CG, Fazzi GE, Blanco CE & De Mey JG (2000). Chronic hypoxia stimulates periarterial sympathetic nerve development in chicken embryo. Circulation 102, 2892–2897. [DOI] [PubMed] [Google Scholar]
- Salinas CE, Blanco CE, Villena M, Camm EJ, Tuckett JD, Weerakkody RA, Kane AD, Shelley AM, Wooding FB, Quy M & Giussani DA (2010). Cardiac and vascular disease prior to hatching in chick embryos incubated at high altitude. J Dev Orig Health Dis 1, 60–66. [DOI] [PubMed] [Google Scholar]
- Samangaya RA, Mires G, Shennan A, Skillern L, Howe D, McLeod A & Baker PN (2009). A randomised, double‐blinded, placebo‐controlled study of the phosphodiesterase type 5 inhibitor sildenafil for the treatment of preeclampsia. Hypertens Pregnancy 28, 369–382. [DOI] [PubMed] [Google Scholar]
- Sanchez‐Aparicio P, Mota‐Rojas D, Nava‐Ocampo AA, Trujillo‐Ortega ME, Alfaro‐Rodriguez A, Arch E & Alonso‐Spilsbury M (2008). Effects of sildenafil on the fetal growth of guinea pigs and their ability to survive induced intrapartum asphyxia. Am J Obstet Gynecol 198, 127.e1–127.e6. [DOI] [PubMed] [Google Scholar]
- Satterfield MC, Bazer FW, Spencer TE & Wu G (2010). Sildenafil citrate treatment enhances amino acid availability in the conceptus and fetal growth in an ovine model of intrauterine growth restriction. J Nutr 140, 251–258. [DOI] [PubMed] [Google Scholar]
- Sharma SK, Lucitti JL, Nordman C, Tinney JP, Tobita K & Keller BB (2006). Impact of hypoxia on early chick embryo growth and cardiovascular function. Pediatr Res 59, 116–120. [DOI] [PubMed] [Google Scholar]
- Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ & Klenk DC (1985). Measurement of protein using bicinchoninic acid. Anal Biochem 150, 76–85. [DOI] [PubMed] [Google Scholar]
- Spickett CM (2013). The lipid peroxidation product 4‐hydroxy‐2‐nonenal: Advances in chemistry and analysis. Redox Biol 1, 145–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley JL, Andersson IJ, Poudel R, Rueda‐Clausen CF, Sibley CP, Davidge ST & Baker PN (2012). Sildenafil citrate rescues fetal growth in the catechol‐O‐methyl transferase knockout mouse model. Hypertension 59, 1021–1028. [DOI] [PubMed] [Google Scholar]
- Thakor AS, Herrera EA, Seron‐Ferre M & Giussani DA (2010. a). Melatonin and vitamin C increase umbilical blood flow via nitric oxide‐dependent mechanisms. J Pineal Res 49, 399–406. [DOI] [PubMed] [Google Scholar]
- Thakor AS, Richter HG, Kane AD, Dunster C, Kelly FJ, Poston L & Giussani DA (2010. b). Redox modulation of the fetal cardiovascular defence to hypoxaemia. J Physiol 588, 4235–4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson LP & Al‐Hasan Y (2012). Impact of oxidative stress in fetal programming. J Pregnancy 2012, 582748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Dadelszen P, Dwinnell S, Magee LA, Carleton BC, Gruslin A, Lee B, Lim KI, Liston RM, Miller SP, Rurak D, Sherlock RL, Skoll MA, Wareing MM, Baker PN for Research into Advanced Fetal Diagnosis & Therapy Group (2011). Sildenafil citrate therapy for severe early‐onset intrauterine growth restriction. BJOG 118, 624–628. [DOI] [PubMed] [Google Scholar]
- Walker DK, Ackland MJ, James GC, Muirhead GJ, Rance DJ, Wastall P & Wright PA (1999). Pharmacokinetics and metabolism of sildenafil in mouse, rat, rabbit, dog and man. Xenobiotica 29, 297–310. [DOI] [PubMed] [Google Scholar]
- Welinder C & Ekblad L (2011). Coomasie staining as lading control in Western blot analysis. J Proteome Res 10, 1416–1419. [DOI] [PubMed] [Google Scholar]
- Wong SL, Leung FP, Lau CW, Au CL, Yung LM, Yao X, Chen ZY, Vanhoutte PM, Gollasch M & Huang Y (2009). Cyclooxygenase‐2‐derived prostaglandin F2alpha mediates endothelium‐dependent contractions in the aortae of hamsters with increased impact during aging. Circ Res 104, 228–235. [DOI] [PubMed] [Google Scholar]


