Figure 3.
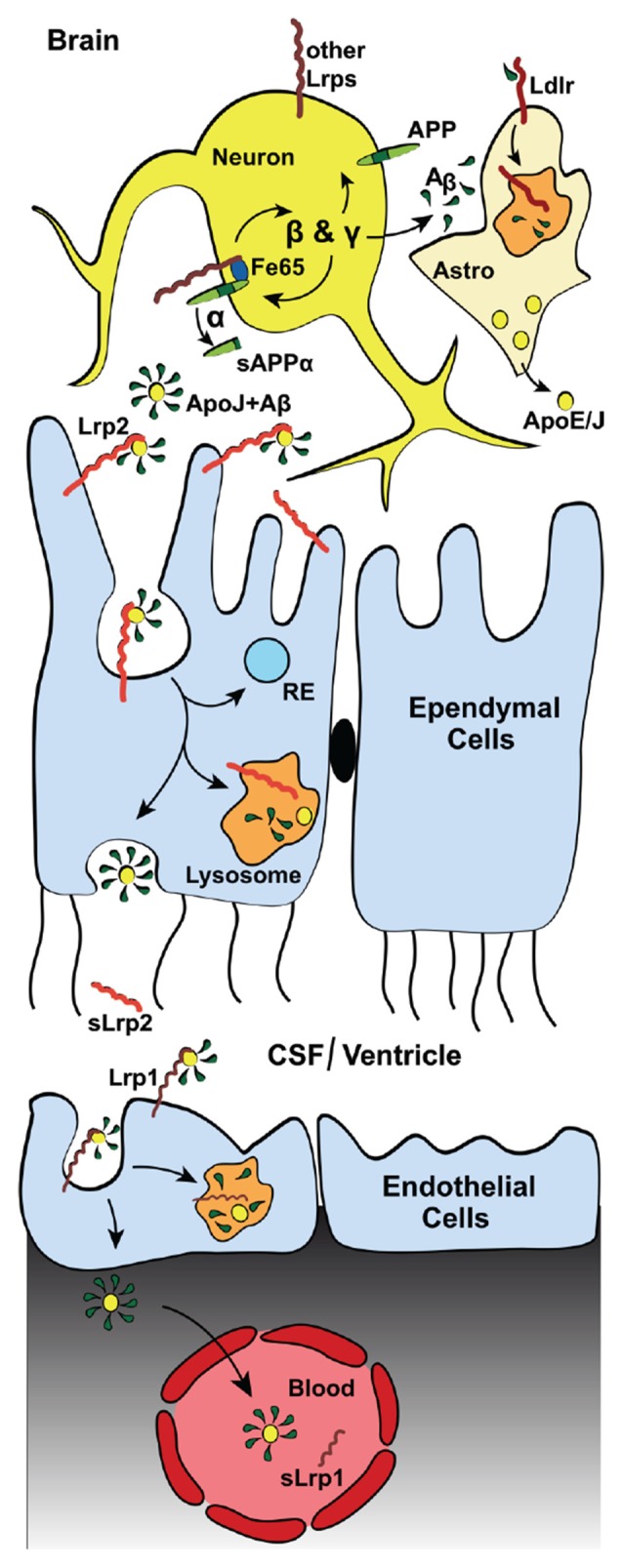
Lrp2 mediates Aβ-clearance via the blood cerebrospinal fluid (CSF) barrier (BCSFB). Diagram depicting the Lrp2-mediated clearance of interstitial Aβ through the cerebral spinal fluid (CSF) into the blood. In addition to direct astrocytic Lrp2 clearance of Aβ, Lrp2 expressed in the ependymal cells of the choroid plexus also facilitate Aβ removal. The choroid plexus functions to produce and filter CSF. This filtration removes metabolic waste, excess neurotransmitters and foreign/toxic particles, such as Aβ, which is mainly produced by neurons (see Figure 2). Apolipoproteins, such as ApoE and ApoJ/Clusterin (yellow dots), mainly secreted from astrocytes (“Astro”), bind circulating interstitial Aβ. These Aβ-laden apolipoproteins then bind lipoprotein receptors (red) and mediate their cellular uptake. ApoJ/Clusterin is eliminated rapidly across the BCSFB by ependymal Lrp2 (light red), facilitating the clearance of Aβ via lysosomal degradation in ependymal cells and subsequent exocytosis into the CSF, where soluble Lrp2 (sLrp2) has been detected (Spuch et al., 2015). BACE1 is the enzyme that processes Lrp2 and Lrp1 to release sLrp2 and sLrp1, respectively. BACE1 is also found in the choroid plexus (Crossgrove et al., 2007; Liu et al., 2013). Other lipoprotein receptors (dark red, most notably Lrp1) then transport Aβ and the apolipoproteins across the endothelial cells from the CSF to the blood vessels of the choroid plexus. sLrp1 can also be detected in plasma, albeit its origin there is mainly peripheral.
