Figure 2. Sorting factors involved in the sequestration of protein aggregates toward quality control sites.
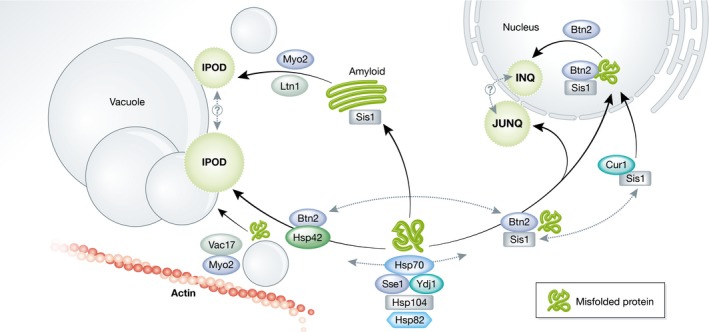
Misfolded and aggregated proteins are recognized by the yeast chaperone machinery: Hsp70s, Hsp40s (Ydj1 & Sis1), the Hsp90 Hsp82, and the disaggregase Hsp104. These chaperones are important for sequestering aggregated proteins into quality control sites: the juxtanuclear JUNQ, intranuclear INQ, and the perivacuolar IPOD sites. Aggregates bound by Btn2 and Sis1 are distributed toward JUNQ and/or INQ. The availability of Sis1 in the cytosol is regulated through competitive binding by Cur1, which translocates a fraction of the cytosolic Sis1 pool into the nucleus, making it unavailable for Btn2 binding. Inside the nucleus Btn2 acts as an aggregase, directing aggregated proteins into the intranuclear INQ inclusion. Btn2 also interacts with the small heat shock protein Hsp42 that is necessary for binding and targeting aggregates to the IPOD. Inclusion formation requires the actin cytoskeleton and vesicle trafficking, which is regulated by the vacuolar adaptor Vac17 and the motor protein Myo2. Amyloidogenic proteins, such as Htt103QP, are sequestered to the IPOD in a pathway independent of Hsp42 and require Sis1 and the E3 ligase Ltn1. This pathway also depends on Myo2‐associated vesicle trafficking, but does not require Vac17. The IPOD formed by amyloids might therefore be distinct from that formed by amorphous aggregates; however, the formation of amyloid IPODs has been shown to influence the formation and dynamics of amorphous inclusions in an up till now undefined manner. See text for more details.
