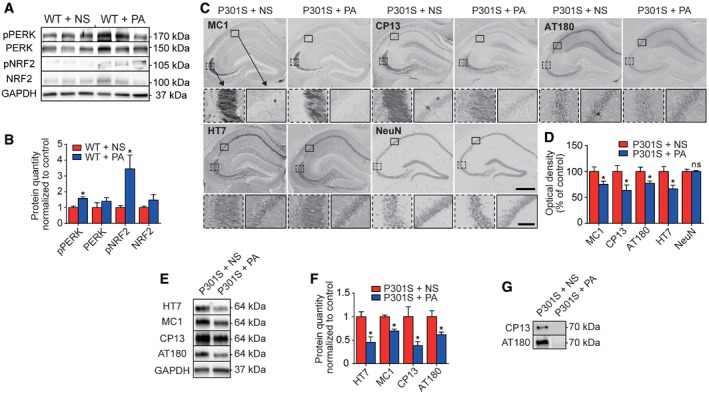
Wild‐type (WT) mice were treated i.p. with normal saline (NS) as control or PERK activator (PA, 2 mg/kg) once daily for 6 weeks. Western blots prepared from brain homogenates. GAPDH was used as loading control.
Densitometric analysis of Western blots described in (A), normalized to WT + NS (n = 3 per condition).
Representative photomicrographs of hippocampi from 23‐week‐old P301S tau transgenic mice, treated as described in (A). Sections were immunostained with antibodies against conformationally changed tau (MC1), phosphorylated tau (CP13, AT180), total tau (HT7), and neuronal nuclei (NeuN). Scale bars, upper: 500 μm, lower: 50 μm.
Optical density measurement of the CA1/CA2 and CA3 region, as shown in (C) (n = 6). Analysis is based on DAB stainings, since this avoids bias by secondary bleaching due to differential storage temperatures/light exposures seen with immunofluorescence.
Representative Western blots of soluble protein fractions from whole‐brain homogenates of mice, treated as in (C).
Densitometric analysis of Western blots as shown in (E), normalized to P301S + NS (n = 3).
Representative Western blots of sarkosyl‐insoluble protein fractions extracted from brain homogenates as in (E), with CP13 and AT180 antibodies.
Data information: Data are mean + SEM. Statistical analysis in (B, D, F) was Student's
0.05 versus control, ns: not significant.