Abstract
Objective:
Antioxidants play a major role in the cellular protection cascade against oxidative damage. Oxidative stress has been linked to the pathogenesis of coronary atherosclerosis. Our aim was to evaluate the association between calculated serum total antioxidant status (cTAS) and the presence and severity of coronary artery disease (CAD).
Methods:
One hundred and seventy-four patients with angiographically documented significant (≥50%) luminal stenosis (n=123) or with minimal (<50%) luminal stenosis (n=51) in at least one coronary artery or major branch segment in the epicardial coronary tree were categorized as CAD+ group; 88 patients with no luminal stenosis were considered as the control group. The level of cTAS (mmol/L) was evaluated using the following equation: (0.63´albumin concentration)+(1.02´uric acid concentration)+(1.53´bilirubin concentration).
Results:
In univariate analyses, mean levels of cTAS, uric acid, and creatinine were significantly higher in CAD+ group than in controls. However, adjusted cTAS level was not found to be a CAD predictor in the total population [odds ratio (OR)=1.20; 95% confidence interval (CI): 0.81–1.76; p=0.364] or in men (OR=1.25; 95% CI: 0.73–2.12; p=0.420) and women (OR=1.20; 95% CI: 0.66–2.19; p=0.553). A weak but statistically significant correlation was found between cTAS and Gensini score (Spearman’s r=0.16, p=0.015).
Conclusion:
In patients with suspicious CAD, the level of cTAS was not found to be an independent predictor for the presence of CAD. Further studies with larger sample size are required to confirm the results.
Keywords: calculated total antioxidant status, coronary angiography, coronary artery disease, Gensini score
Introduction
Coronary artery disease (CAD) is among the leading causes of death worldwide. Traditional and established risk factors of atherosclerosis, such as hypertension, hyperlipidemia, diabetes, age, obesity, and cigarette smoking, play a major role in a person’s chances of developing CAD. However, approximately half of the patients with CAD do not have any of the established risk factors, indicating that other potential CAD risk factors are yet to be identified (1). Over the last decade, several studies have linked the excessive generation of reactive oxygen species (ROS) and resulting oxidative stress with the pathogenesis of coronary atherosclerosis (2–4).
Antioxidants, including various agents such as enzymes (glutathione peroxidase, superoxide dismutase, and catalase), large molecules (albumin and ferritin), and small molecules (uric acid, glutathione, bilirubin, vitamin C, and vitamin E), play an important role in the cellular protection cascade against oxidative damage (5). The total antioxidant status (TAS) mirrors the activity potential of the antioxidant system. Several methods have been introduced to measure the total antioxidant capacity (TAC) in different biological specimens (6). The measurement of TAC reflects the antioxidative status of plasma because antioxidative effects of the plasma antioxidant components are additive (7). The assessment of plasma TAC can be more useful than the measurement of individual antioxidant levels in cells and plasma because it could determine the synergistic interaction among different individual antioxidants (8, 9).
We hypothesized that there is a relationship between coronary occlusion and TAS. The aim of the present study was to investigate the levels of calculated serum total antioxidant status (cTAS) in patients with angiographically documented CAD as compared with those in the control group.
Methods
Study design and population
This cross-sectional study consisted of 262 consecutive patients (187 men, 71.4%), who underwent elective coronary angiography at our institution from December 2010 to July 2012 because of symptoms related to CAD. Patients with acute illness or with a history of renal failure; heart failure; liver and hematologic disease; inflammatory and rheumatic disease, particularly gout; alcohol use; and malignancy as well as those on antioxidant drugs including aspirin, beta blockers, and statins or any drug affecting uric acid, bilirubin, or albumin serum concentrations were excluded. The study protocol was approved by the Ethics Committee of Tehran Heart Center (Approval number: 2011/08/0490) affiliated to Tehran University of Medical Sciences, and written informed consent was achieved from all patients who approved the collection of blood samples for scientific research. Patients with angiographically documented significant (≥50%) luminal stenosis (n=123) or with minimal (<50%) luminal stenosis (n=51) in at least one coronary artery or major branch segment in the epicardial coronary tree were categorized as CAD+ group. Patients with no luminal stenosis (n=88) were considered as the control group.
Anthropometric indices, physical examination, and definitions of CAD risk factors
Height, waist circumference, and weight were measured to the nearest 0.1 cm and 0.1 kg, respectively, by qualified and trained staff. Body mass index was calculated as weight (kg) divided by height squared (m2). The participants remained at rest for at least 10 min and then the staff measured blood pressure using standard calibrated sphygmomanometers. Two measurements were performed for each patient, with at least 1 min interval, and the mean of the two measurements was reported as the patient’s blood pressure. Definitions for analyzed risk factors of CAD have been previously reported (10, 11). In brief, hyperlipidemia was defined as plasma total cholesterol level ≥200 mg/dL and/or low density lipoprotein (LDL)-cholesterol level ≥130 mg/dL or being on lipid-lowering drugs at the time of the study. Patients were considered to have hypertension if they had arterial blood pressure >140/90 mm Hg, or they were being treated with antihypertensive drugs. Patients were considered to have diabetes if they were taking plasma glucose-lowering drugs, including insulin and oral tablets, or had a previous history of diabetes. Patients unaware of their previous histories of diabetes were defined as those who meet the new World Health Organization criteria for diagnosing diabetes mellitus.
Coronary angiography
Coronary angiography was performed using standard angiographic techniques from the percutaneous femoral approach. The angiograms were categorized as either showing no coronary lesions (Absent), no coronary lesions with >50% luminal stenosis (Minimal) or as having one (Mild), two (Moderate), or three (Severe) major epicardial coronary arteries with >50% luminal obstructions. Left main stem (LMS) stenosis was regarded as one vessel. If LMS and the left anterior descending and/or left circumflex arteries were affected, this was counted as two points. The degree of stenosis was visually determined by comparing the greatest percentage reduction of luminal diameter in any view with the nearest normal segment. Gensini score was used for the measurement of CAD severity. This severity score has been previously described (12). In brief, the coronary arterial tree was divided into segments with multiplying factors according to geographic functional importance of any given segment (5 for the left main stem to 0.5 for the most distal segments) as well as the percentage reduction in lumen diameter. The roentgenographic appearance of concentric lesions and eccentric plaques was assigned a score (0, 1, 2, 4, 8, 16, or 32 according to the degree of luminal stenosis). The sum of the segmental scores gives the Gensini score which puts emphasis on the severity of the disease.
Biochemical tests
After an overnight fasting of 10 h, venous blood samples from participants were collected from an antecubital vein in plain tubes and immediately used for biochemical analyses. Biochemical measurements, such as total cholesterol, LDL-cholesterol, high density lipoprotein-cholesterol, triglycerides, and fasting blood sugar levels, were performed with an auto analyzer (Beckman Synchron CX4, Beckman Coulter Inc., Fullerton, CA, USA) using standard methods and commercial kits. All samples were continuously processed. Assay performance was monitored after every 50 tests using the lipid control serum commercial kit (Pars Azmon Inc., Tehran, Iran). Quality control data were plotted on Levey–Jennings chart, and Westgard rules were applied to determine whether the results from the samples can be released, or if they need to be rerun.
Albumin and uric acid were colorimetrically measured using the bromocresol green and uricase, respectively; serum bilirubin level was measured by the diazo method. The plasma concentrations of albumin, bilirubin, and uric acid were measured in mg/dL then converted to mmol/L using related molecular weights. To determine the serum TAS, cTAS (mmol/L) was evaluated using the following equation: (0.63´albumin concentration)+(1.02´uric acid concentration)+(1.53´bilirubin concentration) (13), wherein the concentrations of albumin, uric acid, and bilirubin must be expressed in mmol/L.
As recommended by the American Heart Association (14), we used eGFR (estimated glomerular filtration rate) with the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula rather than serum creatinine to assess renal function.
CKD-EPI equation is expressed as a single equation (15):
eGFR=141´min (Scr/k, 1)a´max (Scr/k, 1)–1.209´(0.993)Age ´1.018 (female)´1.159 (African descent)
Where:
Scr is serum creatinine in mg/dL,
k is 0.7 for females and 0.9 for males,
a is –0.329 for females and –0.411 for males,
min indicates the minimum of Scr/k or 1, and
max indicates the maximum of Scr/k or 1.
Statistical methods
Continuous data were presented as mean with standard deviation and categorical variables were expressed as frequency (%). Categorical variables were compared using chi-square test or Fisher exact test, as appropriate. To assess normality of data distributions, box-plots were visually inspected, and the Kolmogorov–Smirnov normality test was used. In addition, measures of mean, median, skewness, and kurtosis were used to test deviation from normality. When normal distribution was rejected, statistics were calculated using non-parametric test equivalents. Continuous data were compared between CAD+ and non-CAD groups or subgroups using Student’s t-test or Mann–Whitney U test. Among the quartiles of Gensini score, cTAS levels were compared using one-way analysis of variance test. Levene’s test was used to appraise the homogeneity of variances. Spearman’s correlation coefficient was used for calculating the linearity degree between serum cTAS and Gensini score.
Association of cTAS levels with other covariates was measured using Pearson’s or Spearman’s correlation coefficient. Variables that were simultaneously associated with cTAS and CAD with p<0.1 were considered as potential confounders; the effect of cTAS on CAD, adjusted for detected possible confounders, was assessed using binary logistic regression analysis. p≤0.05 was considered statistically significant. All statistical analyses were performed using the SPSS software version 18.0 for windows (SPSS Inc., Chicago, IL, USA).
Results
The mean age of the study patients was 54.4±10.6 years and 187 (71.4%) were men. Overall, the cardiovascular risk factors including hypertension, diabetes mellitus, cigarette smoking, hyperlipidemia, and family history of CAD were common in our study population. Cigarette smoking was much more common in men than in women (32.6% vs. 0, p<0.001). The serum levels of creatinine, uric acid, and cTAS were significantly higher in men than in women (0.9±0.2 vs. 0.7±0.1, 7.6±2.1 vs. 6.8±2.3, and.0.4±0.0 vs. 0.3±0.1, respectively; p<0.001).
The clinical and laboratory characteristics of the participants with and without CAD in total and individually in men and women are presented in Table 1. Mean levels of cTAS, uric acid, and creatinine were significantly higher in CAD patients than in those without CAD. However, after stratification of the study groups by gender, there were no significant differences in men and women subgroups in this regard (p>0.1).
Table 1.
Clinical and laboratory characteristics of patients with and without CAD in whole and in subgroups separated by gender
| All patients (n=262) | Female patients (n=75) | Male patients (n=187) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CAD–(n=88) | CAD+(n=174) | P | CAD–(n=37) | CAD+(n=38) | P | CAD–(n=51) | CAD+(n=136) | P | ||
| Age, years | 49.20±10.70 | 56.95±9.53 | <0.001 | 51.32±8.78 | 55.39±8.68 | 0.047 | 47.67±11.75 | 57.38±9.74 | <0.001 | |
| Male sex | 51 (58.0) | 136 (78.2) | 0.001 | – | – | – | – | – | – | |
| BMI, kg/m2 | 27.36±4.55 | 28.04±4.25 | 0.237 | 29.26±4.65 | 30.01±4.34 | 0.471 | 25.98±3.99 | 27.48±4.07 | 0.026 | |
| Cigarette smoking | 0.036 | 0.999 | 0.356 | |||||||
| Current smoker | 17 (19.3) | 44 (25.3) | 0 | 0 | 17 (33.3) | 44 (32.4) | ||||
| Ex-smoker | 7 (8.0) | 30 (17.2) | 1 (2.7) | 2 (5.3) | 6 (11.8) | 28 (20.6) | ||||
| Non-smoker | 64 (72.7) | 100 (57.5) | 36 (97.3) | 36 (94.7) | 28 (54.9) | 64 (47.1) | ||||
| Hypertension | 27 (30.7) | 78 (44.8) | 0.027 | 13 (35.1) | 19 (50.0) | 0.193 | 14 (27.5) | 59 (43.4) | 0.047 | |
| Diabetes | 4 (4.5) | 36 (20.7) | 0.001 | 1 (2.7) | 12 (31.6) | 0.001 | 3 (5.9) | 24 (17.6) | 0.041 | |
| Hyperlipidemia | 39 (44.3) | 117 (67.2) | <0.001 | 20 (54.1) | 23 (60.5) | 0.571 | 19 (37.3) | 94 (69.1) | <0.001 | |
| FH of CAD | 8 (9.1) | 25 (14.4) | 0.223 | 6 (16.2) | 7 (18.4) | 0.759 | 2 (4.0) | 18 (13.2) | 0.067 | |
| FBS, mg/dL | 102.93±36.77 | 119.23±43.39 | 0.002 | 109.72±54.57 | 135.92±54.48 | 0.045 | 98.14±13.58 | 114.61±38.79 | <0.001 | |
| Gensini score | 0 | 28.5 (8 to 59) | <0.001 | 0 | 15.0 (7–51) | 0.001 | 0 | 32.5 (8–64) | <0.001 | |
| LVEF, % | 54.30±5.90 | 50.79±9.28 | 0.002 | 54.93±5.01 | 49.82±11.34 | 0.035 | 53.82±6.52 | 51.07±8.62 | 0.080 | |
| cTAS, mmol/L | 0.37±0.89 | 0.39±0.93 | 0.050 | 0.32±0.85 | 0.34 ± 0.11 | 0.457 | 0.40±0.80 | 0.41±0.84 | 0.526 | |
| Albumin, g/dL | 4.78±0.30 | 4.72±0.31 | 0.184 | 4.71±0.30 | 4.68±0.31 | 0.679 | 4.82±0.29 | 4.73±0.31 | 0.087 | |
| Bilirubin, mg/dL | 0.55±0.36 | 0.56±0.34 | 0.428 | 0.46±0.26 | 0.46±0.25 | 0.967 | 0.61±0.40 | 0.59±0.36 | 0.705 | |
| ALT, u/L | 12.66±10.89 | 13.37±10.03 | 0.317 | 10.40±8.69 | 11.29±6.93 | 0.629 | 14.27±12.05 | 13.99±10.72 | 0.882 | |
| AST, u/L | 18.95±8.01 | 20.61±13.26 | 0.271 | 16.80±4.96 | 16.97±5.73 | 0.891 | 20.49±9.37 | 21.70±14.62 | 0.593 | |
| Creatinine, mg/dL | 0.80±0.17 | 0.89±0.21 | 0.001 | 0.67±0.11 | 0.69±0.15 | 0.525 | 0.89±0.15 | 0.94±0.19 | 0.143 | |
| eGFR, ml/min/1.73 | 99.52±14.02 | 91.35±16.06 | <0.001 | 98.92±12.27 | 93.90±14.75 | 0.121 | 99.97±15.29 | 90.66±16.38 | 0.001 | |
| Uric acid, mg/dL | 5.75±1.44 | 6.14±1.51 | 0.033 | 5.09±1.38 | 5.37±1.72 | 0.444 | 6.21±1.30 | 6.37±1.37 | 0.491 | |
Data are presented as mean±standard deviation or n (%) except for Gensini score which is presented as median (25th to 75th percentiles)
ALT - alanine transaminase; AST - aspartate transaminase; BMI - body mass index; CAD - coronary artery disease; cTAS - calculated total antioxidant status; eGFR - estimated glomerular filtration rate; FBS - fasting blood glucose; FH - family history; LVEF - left ventricular ejection fraction
Spearman’s correlation coefficient demonstrated a weak but statistically significant correlation between the serum cTAS level and the severity of CAD by using Gensini score (Spearman’s r=0.16, p=0.015). Moreover, mean cTAS levels in different Gensini score quartiles were tested and revealed no significant difference (Fig. 1).
Figure 1.
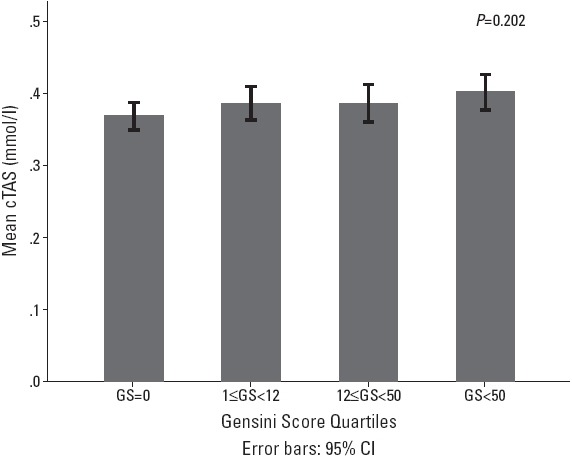
The association between different Gensini score quartiles and serum cTAS level; cTAS, calculated total antioxidant score; GS, Gensini score
In a multiple logistic regression model, adjusted effects of cTAS levels on CAD presence were assessed. As shown in Table 2, after adjusting for other covariates, cTAS level was not found to be an independent predictor for CAD occurrence in the total population (OR=1.20; 95% CI: 0.81–1.76; p=0.364) or in men [OR=1.25; 95% confidence interval (CI): 0.73–2.12; p=0.420] and women (OR=1.20; 95% CI: 0.66–2.19; p=0.553). We did not find any significant difference in cTAS levels between CAD+ and non-CAD groups and were unable to find a cut-off for cTAS measure to predict the presence or severity of CAD.
Table 2.
Binary logistic regression model for identifying the independent effect of cTAS level on CAD in all participants as well as in male and female participants
| All participants | Male | Female | ||||
|---|---|---|---|---|---|---|
| Predictors | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P |
| cTAS, per 0.1 mmol/L increase | 1.2 (0.8–1.8) | 0.375 | 1.3 (0.7–2.1) | 0.395 | 1.2 (0.7–2.2) | 0.548 |
| Age, years | 1.1 (1.0–1.1) | <0.001 | 1.1 (1.0–1.1) | 0.001 | 1.1 (0.9–1.1) | 0.248 |
| Male sex | 2.5 (1.1–6.5) | 0.030 | – | – | – | – |
| Hypertension | 1.2 (0.6–2.4) | 0.609 | 1.3 (0.5–3.0) | 0.599 | 1.6 (0.5–5.0) | 0.438 |
| Hyperlipidemia | 2.1 (1.1–3.9) | 0.025 | 3.1 (1.4–6.9) | 0.004 | 1.0 (0.3–3.2) | 0.935 |
| Cigarette use | 0.699 | 0.727 | 0.738 | |||
| Non-smoker | 1* | – | 1* | – | 1* | – |
| Ex-smoker | 1.1 (0.4–3.2) | 0.887 | 1.1 (0.3–3.5) | 0.907 | 1.7 (0.9–30.8) | 0.738 |
| Current smoker | 1.4 (0.6–3.3) | 0.402 | 1.4 (0.6–3.5) | 0.434 | – | – |
| FH of CAD | 2.1 (0.7–5.7) | 0.172 | 3.6 (0.7–18.6) | 0.120 | 1.3 (0.3–6.2) | 0.752 |
| Diabetes | 5.2 (1.6–16.8) | 0.006 | 2.1 (0.5–8.4) | 0.280 | 20.4 (2.1–198.3) | 0.009 |
| eGFR | 1.0 (0.9–1.0) | 0.397 | 1.0 (0.9–1.0) | 0.833 | 1.0 (0.9–1.0) | 0.292 |
Reference group
CAD - coronary artery disease; CI - confidence interval; cTAS - calculated total antioxidant status; eGFR - estimated glomerular filtration rate; FBS - fasting blood glucose; FH - family history; OR - odds ratio
Discussion
The main finding of the present study is that the mean cTAS level in the CAD+ group was significantly higher than that in the control group in univariate analyses, but not after adjusting for other covariates, such as age, sex, hyperlipidemia, and diabetes mellitus. ROS are part of unspecified defense system of various organisms. However, excessive ROS levels may cause cellular damage, resulting in oxidative stress from an imbalance in the ratio of ROS production to degradation. The role of oxidative stress in the development of CAD is well known (16). Several previous investigators have reported that oxidative stress is associated with vulnerable plaque and occurrence of acute coronary syndrome (17–20). Results from clinical trials using antioxidant supplementation for improving cardiovascular outcomes in humans have, however, not been as promising as expected (21, 22), and whether antioxidant interventions actually succeeded in decreasing ROS levels and oxidative stress was never ascertained (23).
The evaluation of a single parameter may cause misinterpretations, and oxidative stress should be evaluated as a whole including both total oxidative stress and total antioxidant capacity. Although the combined antioxidant activity of albumin, bilirubin, and uric acid does not necessarily reflect the whole scenario of antioxidant capacity, it majorly contributes to the total antioxidant activity in plasma. Although patients with acute disease were not included in the study, the interval between the onset of symptoms and angiographic intervention could have affected oxidative status, and it would have been much better to investigate new patients who had just started to show symptoms.
Each measure of antioxidant status has its own limitations. Direct measurement of ROS has been described, but these species are transient in nature; the procedure is complex, and the results have not always shown to be reliable (24–26). The methods for calculating measured TAS (mTAS), measurement of TAS levels in the plasma using a spectrophotometer, are relatively inexpensive and usually straightforward. However, colorimetry as one of the most widely used methods for measuring total oxidant status involves either fluorescence or chemiluminescence, which requires sophisticated techniques; these technologies are unavailable in many routine clinical biochemistry laboratories, or even if available, their routine use is limited (27). In order to detect the antioxidative status of plasma, instead of mTAS, we calculated TAS using Bonnefont-Rousselot et al. (13) method that is an adaptation of a method initially described by Miller et al. (28). We chose this method because it is an easier and cheaper method (or formula), and as Lassnigg et al. (29) stated, albumin, uric acid, and total bilirubin are robust markers for assessing TAS.
Our finding that TAS levels were significantly higher in univariate analyses in CAD patients than in the control group may be explained by higher levels of uric acid and other antioxidants in these patients. Indeed, cTAS is calculated based on uric acid, bilirubin, and albumin concentrations in the serum. In a previous study, high uric acid level was found to be independently associated with the development of CAD (11); however, it is still unknown whether high serum uric acid is causally an independent risk factor, a consequence, or merely a marker for CAD (30). Another finding of our study is that the level of cTAS was positively but negligibly correlated with Gensini score. Several other studies suggested that the severity of coronary atherosclerosis calculated by Gensini score was positively correlated with oxidative stress markers (31–33).
Because cigarette smoking, renal function, and diabetes mellitus are important conditions of increased oxidative stress that should be addressed (34, 35), multiple logistic regression models were established to compare outcome variables across groups, with the presence of other covariates, to find the independent effect of cTAS on the presence and severity of CAD. The level of cTAS was not found to be an independent predictor for CAD occurrence. Consistent with our result, the TAS level showed no significant independent contribution to CAD among middle-aged men in a study by Nojiri et al. (36). Several other studies also found no significant difference in TAS levels between CAD patients and control group (37–39). However, the relationship between CAD and TAS is controversial. In a recent study, Aydın et al. (31) showed that in patients with CAD, the plasma TAC levels were increased; they ascribed increased TAC levels to the use of drugs with anti-oxidative impacts or to the intake of dietary antioxidant nutrients in patients with severe CAD. Nieto et al. (30) also studied 150 subclinical cases of carotid atherosclerosis identified by carotid ultrasound and reported that the levels of serum TAC were significantly higher than in controls; they almost entirely explained this difference by the rise in serum uric acid level in the case group. In contrast, Fazendas et al. (40) demonstrated that plasma TAS was decreased in patients with myocardial infarction; numerous other studies have also shown a significant reduction in the level of TAC among CAD patients (41–46). Such disparity in study results may be because of variation in study design, small sample sizes, age, gender, and differences in other genetic and environmental risk factors among various populations.
The estimated renal function in our patients with CAD was worse than in the control group in univariate analysis; however, multiple regression models showed that the association between eGFR and CAD was strongly affected by confounding variables. The levels of different markers of oxidative stress have been shown to increase in patients with different degrees of renal function, including patients with end-stage renal failure (47, 48). It has been suggested that the level of renal function significantly correlates with TAS; however, it appears to be dependent on several confounding variables, including increased uric acid levels (47). On the other hand, Karamouzis et al. (48) suggested that antioxidant capacity remains rather stable with the loss of renal function and changes only in patients with end-stage renal failure.
Study limitations
The results of the present study should be interpreted with caution because in our study, TAS level was merely calculated (i.e., cTAS) and this equation is neither very sensitive nor specific. In addition, the serum TAS measured or calculated in circulating blood cannot necessarily reflect antioxidant concentrations in atherosclerotic plaques as the target tissue. In addition, a history of coronary revascularization and presence of collaterals could influence oxidant/anti-oxidant balance in CAD patients. But related data were not available. Finally, the small sample size that restricts the power to detect associations with statistical analyses may be considered a potential limit of this study. Because a large percentage of patients with CAD were on antioxidant medications and excluded from our study, this should be considered a relatively large sample size; however, the results need to be confirmed in larger studies.
Conclusion
Our findings demonstrated that serum cTAS level was significantly associated with the presence of CAD in univariate analyses. However, after controlling for other covariates, the level of cTAS was not found to be an independent predictor for CAD. Further studies with larger sample size are required to confirm the results.
Footnotes
Conflict of interest: None declared.
Peer-review: Externally peer-reviewed.
Authorship contributions: Concept- M.S.A.; Design- M.M.B.; Supervision- M.A.B.; Data collection &/or processing – B.E., A.J.; Analysis and/or interpretation– A.J., M.M.B.; Literature search- B.E.;Writing – H.G., M.S.A.; Critical review- M.S.A., M.A.B.
References
- 1.Futterman LG, Lemberg L. Fifty percent of patients with coronary artery disease do not have any of the conventional risk factors. Am J Crit Care. 1998;7:240–4. [PubMed] [Google Scholar]
- 2.Kotur-Stevuljevic J, Memon L, Stefanovic A, Spasic S, Spasojevic-Kalimanovska V, Bogavac-Stanojevic N, et al. Correlation of oxidative stress parameters and inflammatory markers in coronary artery disease patients. Clin Biochem. 2007;40:181–7. doi: 10.1016/j.clinbiochem.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 3.Serdar Z, Aslan K, Dirican M, Sarandol E, Yeşilbursa D, Serdar A. Lipid and protein oxidation and antioxidant status in patients with angiographically proven coronary artery disease. Clin Biochem. 2006;39:794–803. doi: 10.1016/j.clinbiochem.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 4.Kaur K, Bedi G, Kaur M, Vij A, Kaur I. Lipid peroxidation and the levels of antioxidant enzymes in coronary artery disease. Indian J Clin Biochem. 2008;23:33–7. doi: 10.1007/s12291-008-0008-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hsu CH, Chi BC, Liu MY, Li JH, Chen CJ, Chen RY. Phosphine-induced oxidative damage in rats:role of glutathione. Toxicology. 2002;179:1–8. doi: 10.1016/s0300-483x(02)00246-9. [DOI] [PubMed] [Google Scholar]
- 6.Yeum KJ, Russell RM, Krinsky NI, Aldini G. Biomarkers of antioxidant capacity in the hydrophilic and lipophilic compartments of human plasma. Arch Biochem Biophys. 2004;430:97–103. doi: 10.1016/j.abb.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 7.Erel O. A new automated colorimetric method for measuring total oxidant status. Clin Biochem. 2005;38:1103–11. doi: 10.1016/j.clinbiochem.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 8.Kampa M, Nistikaki A, Tsaousis V, Maliaraki N, Notas G, Castanas E. A new automated method for the determination of the Total Antioxidant Capacity (TAC) of human plasma, based on the crocin bleaching assay. BMC Clin Pathol. 2002;2:3. doi: 10.1186/1472-6890-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Erel O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin Biochem. 2004;37:277–85. doi: 10.1016/j.clinbiochem.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 10.Boroumand M, Ghaedi M, Mohammadtaghvaei N, Pourgholi L, Anvari MS, Davoodi G, et al. Association of estrogen receptor alpha gene polymorphism with the presence of coronary artery disease documented by coronary angiography. Clin Biochem. 2009;42:835–9. doi: 10.1016/j.clinbiochem.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 11.Goodarzynejad H, Anvari MS, Boroumand MA, Karimi A, Abbasi SH, Davoodi G. Hyperuricemia and the presence and severity of coronary artery disease. Lab Medicine. 2010;41:40–5. [Google Scholar]
- 12.Gensini GG. A more meaningful scoring system for determining the severity of coronary heart disease. Am J Cardiol. 1983;51:606. doi: 10.1016/s0002-9149(83)80105-2. [DOI] [PubMed] [Google Scholar]
- 13.Bonnefont-Rousselot D, Lehmann E, Jaudon MC, Delattre J, Perrone B, Rechke JP. Blood oxidative stress and lipoprotein oxidizability in haemodialysis patients:effect of the use of a vitamin E-coated dialysis membrane. Nephrol Dial Transplant. 2000;15:2020–8. doi: 10.1093/ndt/15.12.2020. [DOI] [PubMed] [Google Scholar]
- 14.Brosius FC, 3rd, Hostetter TH, Kelepouris E, Mitsnefes MM, Moe SM, Moore MA, et al. Detection of chronic kidney disease in patients with or at increased risk of cardiovascular disease:a science advisory from the American Heart Association Kidney And Cardiovascular Disease Council;the Councils on High Blood Pressure Research, Cardiovascular Disease in the Young, and Epidemiology and Prevention;and the Quality of Care and Outcomes Research Interdisciplinary Working Group:developed in collaboration with the National Kidney Foundation. Circulation. 2006;114:1083–7. doi: 10.1161/CIRCULATIONAHA.106.177321. [DOI] [PubMed] [Google Scholar]
- 15.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soydinç S, Çelik A, Demiryürek S, Davutoğlu V, Tarakçıoğlu M, Aksoy M. The relationship between oxidative stress, nitric oxide, and coronary artery disease. Eur J Gen Med. 2007;4:62–6. [Google Scholar]
- 17.Menteşe U, Doğan OV, Turan I, Usta S, Doğan E, Menteşe SO, et al. Oxidant-antioxidant balance during on-pump coronary artery bypass grafting. ScientificWorldJournal. 2014;2014:263058. doi: 10.1155/2014/263058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sigala F, Kotsinas A, Savari P, Filis K, Markantonis S, Iliodromitis EK, et al. Oxidized LDL in human carotid plaques is related to symptomatic carotid disease and lesion instability. J Vasc Surg. 2010;52:704–13. doi: 10.1016/j.jvs.2010.03.047. [DOI] [PubMed] [Google Scholar]
- 19.Rajagopalan S, Meng XP, Ramasamy S, Harrison DG, Galis ZS. Reactive oxygen species produced by macrophage-derived foam cells regulate the activity of vascular matrix metalloproteinases in vitro. Implications for atherosclerotic plaque stability. J Clin Invest. 1996;98:2572–9. doi: 10.1172/JCI119076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yazıcı S, Demirtaş S, Güçlü O, Karahan O, Yavuz C, Çalışkan A, et al. Using oxidant and antioxidant levels to predict the duration of both acute peripheral and mesenteric ischemia. Perfusion. 2014;29:450–5. doi: 10.1177/0267659114524012. [DOI] [PubMed] [Google Scholar]
- 21.Lonn E, Bosch J, Yusuf S, Sheridan P, Pogue J, Arnold JM, et al. Effects of long-term vitamin E supplementation on cardiovascular events and cancer:a randomized controlled trial. JAMA. 2005;293:1338–47. doi: 10.1001/jama.293.11.1338. [DOI] [PubMed] [Google Scholar]
- 22.Clarke R, Armitage J. Antioxidant vitamins and risk of cardiovascular disease. Review of large-scale randomised trials. Cardiovasc Drugs Ther. 2002;16:411–5. doi: 10.1023/a:1022134418372. [DOI] [PubMed] [Google Scholar]
- 23.Halliwell B. The antioxidant paradox. Lancet. 2000;355:1179–80. doi: 10.1016/S0140-6736(00)02075-4. [DOI] [PubMed] [Google Scholar]
- 24.Clermont G, Vergely C, Jazayeri S, Lahet JJ, Goudeau JJ, Lecour S, et al. Systemic free radical activation is a major event involved in myocardial oxidative stress related to cardiopulmonary bypass. Anesthesiology. 2002;96:80–7. doi: 10.1097/00000542-200201000-00019. [DOI] [PubMed] [Google Scholar]
- 25.Wu ZK, Tarkka MR, Eloranta J, Pehkonen E, Laurikka J, Kaukinen L, et al. Effect of ischaemic preconditioning, cardiopulmonary bypass and myocardial ischaemic/reperfusion on free radical generation in CABG patients. Cardiovasc Surg. 2001;9:362–8. doi: 10.1016/s0967-2109(00)00146-0. [DOI] [PubMed] [Google Scholar]
- 26.Ferreira R, Llesuy S, Milei J, Scordo D, Hourquebie H, Molteni L, et al. Assessment of myocardial oxidative stress in patients after myocardial revascularization. Am Heart J. 1988;115:307–12. doi: 10.1016/0002-8703(88)90475-9. [DOI] [PubMed] [Google Scholar]
- 27.Erel O. A novel automated method to measure total antioxidant response against potent free radical reactions. Clin Biochem. 2004;37:112–9. doi: 10.1016/j.clinbiochem.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 28.Miller NJ, Rice-Evans C, Davies MJ, Gopinathan V, Milner A. A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status in premature neonates. Clin Sci (Lond) 1993;84:407–12. doi: 10.1042/cs0840407. [DOI] [PubMed] [Google Scholar]
- 29.Lassnigg A, Punz A, Barker R, Keznickl P, Manhart N, Roth E, et al. Influence of intravenous vitamin E supplementation in cardiac surgery on oxidative stress:a double-blinded, randomized, controlled study. Br J Anaesth. 2003;90:148–54. doi: 10.1093/bja/aeg042. [DOI] [PubMed] [Google Scholar]
- 30.Nieto FJ, Iribarren C, Gross MD, Comstock GW, Cutler RG. Uric acid and serum antioxidant capacity:a reaction to atherosclerosis? Atherosclerosis. 2000;148:131–9. doi: 10.1016/s0021-9150(99)00214-2. [DOI] [PubMed] [Google Scholar]
- 31.Aydın M, Selcoki Y, Nazlı Y, Çolak N, Yalçın KS, Canbal M, et al. Relationship between total antioxidant capacity and the severity of coronary artery. J Clin Exp Invest www jceionline org Vol. 2012;3 [Google Scholar]
- 32.Turan T, Menteşe U, Ağaç MT, Akyüz AR, Kul S, Aykan AC, et al. The relation between intensity and complexity of coronary artery lesion and oxidative stress in patients with acute coronary syndrome. Anatol J Cardiol. 2015;15:795–800. doi: 10.5152/akd.2014.5761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.DemirbağR Rabus B, Sezen Y, Taşkın A, Kalaycı S. The plasma and tissue oxidative status in patients with coronary artery disease:oxidative stress and coronary artery disease. Turkish J Thorac Cardiovasc Surg. 2010;18:079–82. [Google Scholar]
- 34.Aksoy S, Cam N, Gürkan U, Öz D, Özden K, Altay S, et al. Oxidative stress and severity of coronary artery disease in young smokers with acute myocardial infarction. Cardiol J. 2012;19:381–6. doi: 10.5603/cj.2012.0069. [DOI] [PubMed] [Google Scholar]
- 35.Madamanchi NR, Vendrov A, Runge MS. Oxidative stress and vascular disease. Arterioscler Thromb Vasc Biol. 2005;25:29–38. doi: 10.1161/01.ATV.0000150649.39934.13. [DOI] [PubMed] [Google Scholar]
- 36.Nojiri S, Daida H, Mokuno H, Iwama Y, Mae K, Ushio F, et al. Association of serum antioxidant capacity with coronary artery disease in middle-aged men. Jpn Heart J. 2001;42:677–90. doi: 10.1536/jhj.42.677. [DOI] [PubMed] [Google Scholar]
- 37.Doğru-Abbasoğlu S, Kanbağlı O, Bulur H, Babalık E, Öztürk S, Aykaç-Toker G, et al. Lipid peroxides and antioxidant status in serum of patients with angiographically defined coronary atherosclerosis. Clin Biochem. 1999;32:671–2. doi: 10.1016/s0009-9120(99)00076-4. [DOI] [PubMed] [Google Scholar]
- 38.Markovic S, Dordevic J, Majkic-Singh N, Vasiljevic Z, Petrovic M, Glavinic L, et al. The importance of antioxidant enzyme and total antioxidant status of patients with acute myocardial infarction on thrombolytic therapy. Clin Lab. 2000;46:495–9. [PubMed] [Google Scholar]
- 39.Gawron-Skarbek A, Chrzczanowicz J, Kostka J, Nowak D, Drygas W, Jegier A, et al. Cardiovascular risk factors and total serum antioxidant capacity in healthy men and in men with coronary heart disease. Biomed Res Int. 2014;2014:216964. doi: 10.1155/2014/216964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fazendas P, Joao IF, Llobet S, Matias F, Pereira H, Oliveira LM, et al. Plasma total anti-oxidant status in young survivors of myocardial infarction. Rev Port Cardiol. 2000;19:463–7. [PubMed] [Google Scholar]
- 41.Shaikh AK, Suryakar AN. Oxidative stress and antioxidant status before and after supplementation of AZ anti-oxidant tablets in coronary artery disease. Biomedical Research. 2009;20:136–40. [Google Scholar]
- 42.Khaki-khatibi F, Yaghoubi AR, Rahbani NM. Study of antioxidant enzymes, lipid peroxidation, lipid profile and immunologic factor in coronary artery disease in East Azarbijan. Int J Med Biomed Res. 2012;1:147–52. [Google Scholar]
- 43.Demirbağ R, Yılmaz R, Koçyiğit A. Relationship between DNA damage, total antioxidant capacity and coronary artery disease. Mutat Res. 2005;570:197–203. doi: 10.1016/j.mrfmmm.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 44.Gür M, Aslan M, Yıldız A, Demirbağ R, Yılmaz R, Selek S, et al. Paraoxonase and arylesterase activities in coronary artery disease. Eur J Clin Invest. 2006;36:779–87. doi: 10.1111/j.1365-2362.2006.01727.x. [DOI] [PubMed] [Google Scholar]
- 45.Cavalca V, Veglia F, Squellerio I, Marenzi G, Minardi F, De Metrio M, et al. Glutathione, vitamin E and oxidative stress in coronary artery disease:relevance of age and gender. Eur J Clin Invest. 2009;39:267–72. doi: 10.1111/j.1365-2362.2009.02094.x. [DOI] [PubMed] [Google Scholar]
- 46.Jawalekar SL, Kulkarni UJ, Surve VT, Deshmukh Y. Status of lipid profile, MDA and protein carbonyl in patients with cardiovascular diseases. Arch Appl Sci Res. 2010;2:8–14. [Google Scholar]
- 47.Dounousi E, Papavasiliou E, Makedou A, Ioannou K, Katopodis KP, Tselepis A, et al. Oxidative stress is progressively enhanced with advancing stages of CKD. Am J Kidney Dis. 2006;48:752–60. doi: 10.1053/j.ajkd.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 48.Karamouzis I, Sarafidis PA, Karamouzis M, Iliadis S, Haidich AB, Sioulis A, et al. Increase in oxidative stress but not in antioxidant capacity with advancing stages of chronic kidney disease. Am J Nephrol. 2008;28:397–404. doi: 10.1159/000112413. [DOI] [PubMed] [Google Scholar]


