Abstract
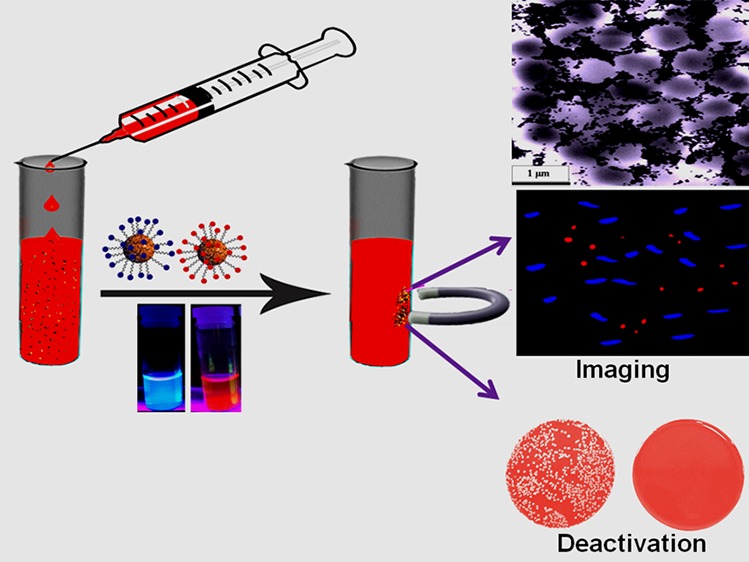
The emergence of drug-resistant superbugs remains a major burden to society. As the mortality rate caused by sepsis due to superbugs is more than 40%, accurate identification of blood infections during the early stage will have a huge significance in the clinical setting. Here, we report the synthesis of red/blue fluorescent carbon dot (CD)-attached magnetic nanoparticle-based multicolor multifunctional CD-based nanosystems, which can be used for selective separation and identification of superbugs from infected blood samples. The reported data show that multifunctional fluorescent magneto-CD nanoparticles are capable of isolating Methicillin-resistant Staphylococcus aureus (MRSA) and Salmonella DT104 superbug from whole blood samples, followed by accurate identification via multicolor fluorescence imaging. As multidrug-resistant (MDR) superbugs are resistant to antibiotics available in the market, this article also reports the design of antimicrobial peptide-conjugated multicolor fluorescent magneto-CDs for effective separation, accurate identification, and complete disinfection of MDR superbugs from infected blood. The reported data demonstrate that by combining pardaxin antimicrobial peptides, magnetic nanoparticles, and multicolor fluorescent CDs into a single system, multifunctional CDs represent a novel material for efficient separation, differentiation, and eradication of superbugs. This material shows great promise for use in clinical settings.
1. Introduction
Center for Global Health predicts that drug-resistant superbugs kill 700 000 people per year and pose a fundamental threat to human health.1−4 World Health Organization indicates that by 2050, superbugs could be responsible for 10 million deaths per year, more than the number of people who die from cancer yearly.1,2 Sepsis due to blood stream infection is one of the major health problems with a mortality rate of more than 40%.1−3 The high mortality rate is mainly due to the absence of technology available in clinics which can rapidly detect and identify bacteria from clinical blood samples in the early stages of infection.3−8 The present “gold standard” used in clinics is bacterial blood cultures for 24–48 h and then susceptibility testing for drug resistance.3−8 The whole process requires several days to obtain accurate results. Because patients need to be treated at the time of the visit, physicians prescribe broad-spectrum antibiotics.6−12 This general approach not only is inadequate to treat patients who have drug-resistant infections but also encourages antibiotic resistance.8−14 Owing to the inability of the current methods to provide accurate results in a short time, new technology that can be used to rapidly diagnose drug-resistant superbugs in a point-of-care setting is needed.1−8 On the basis of this need, this article reports the design of multicolor fluorescent carbon dot (CD)-conjugated magnetic nanoparticle-based multifunctional nanosystem for the selective separation and accurate identification of superbugs from infected blood samples.
Carbon dots (CDs) are quasispherical particles of diameters less than 5 nm, whose surface contains multiple oxygen-containing moieties.15−30 Because they can be produced from inexpensive starting materials in large scale and exhibit remarkably bright multicolor photoluminescence due to quantum confinement effects, CDs hold great promise for daily-life applications.31−41 Similarly, magnetic nanoparticles have been used commonly for the magnetic separation of targeted biological molecules from blood.16,30,42,43 Several recent reports show that the separation of targeted molecules from blood is necessary to minimize light scattering and autofluorescence during imaging.15,16,30,42,43 In this approach, the magnetic properties of the multifunctional CDs have been used for the removal of superbugs from the blood sample, providing effective separation and enrichment, a key step in the diagnosis of superbugs in the early stage of an infection. The CDs can be used to visualize different superbugs via multicolor fluorescence imaging to provide accurate diagnosis. To demonstrate that the multifunctional fluorescent magneto-CDs can be used for the analysis of different types of superbugs in a clinical setting, citrated whole blood samples purchased from Colorado Serum Company were inoculated with a trace level of multidrug-resistant (MDR) strains of Staphylococcus aureus (MRSA) and Salmonella enterica serotype typhimurium definitive phage type 104 (DT104) at different colony-forming unit densities. Experiments have demonstrated that bioconjugated multifunctional fluorescent magneto-CDs are capable of capturing both types of superbugs from the whole blood samples and accurate identification each by multicolor fluorescence imaging.
Because the Salmonella DT104 strain is resistant to several antibiotics, including ampicillin, chloramphenicol/florfenicol, spectinomycin/streptomycin, sulfonamides, and tetracyclines, and also no new antibiotics for this superbug has emerged, the development of alternative antimicrobial agents is urgent.1−8 Antimicrobial peptides are natural and synthetic oligopeptides that are highly promising antimicrobial agents against superbugs by bolstering the host’s defense and modulating the immune response.8−13,38−40 Pardaxin (Gly-Phe-Phe-Ala-Leu-Ile-Pro-Lys-Ile-Ile-Ser-Ser-Pro-Leu-Phe-Lys-Thr-Leu-Leu-Ser-Ala-Val-Gly-Ser-Ala-Leu-Ser-Ser-Ser-Gly-Gly-Gln-Glu) is a well-documented pore-forming peptide with antimicrobial activity against both Gram-positive and Gram-negative bacteria through the disruption of bacterial membranes and the arachidonic acid cascade.12,13 By combining pardaxin antimicrobial peptides, magnetic nanoparticles, and multicolor fluorescent CDs into a single system, the multifunctional CDs represent a novel material for total separation, complete differentiation, and accurate identification of superbugs.
2. Results and Discussion
2.1. Design of Blue and Red Fluorescent Carbon Dots Using Phenylenediamine
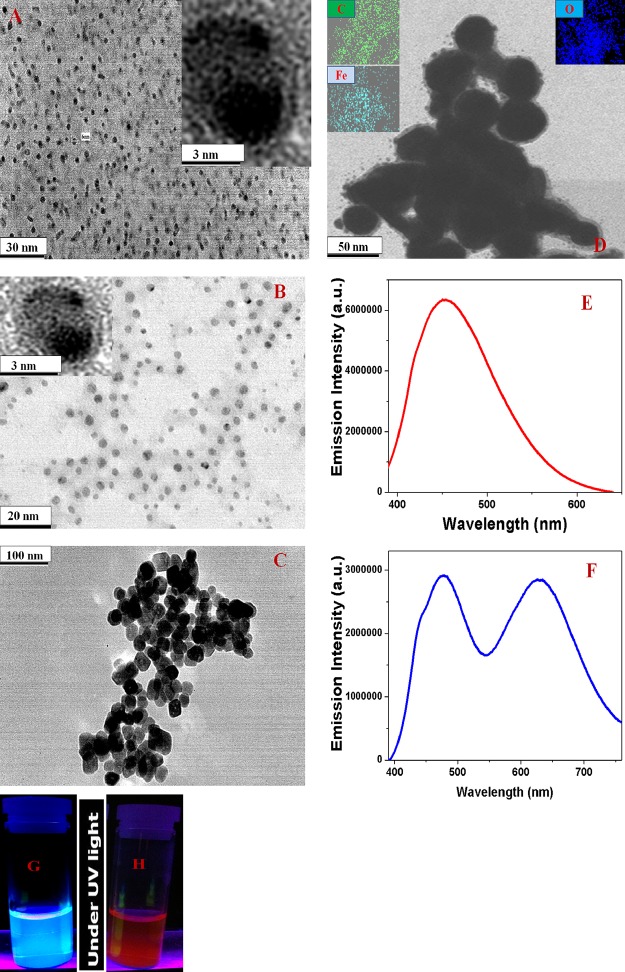
CDs exhibiting strong blue fluorescence were synthesized by hydrothermal heating of meta-phenylenediamine in ethanol using a reported method.26 Similarly, the red fluorescent CDs were synthesized by microwave-assisted heating of para-phenylenediamine using a reported method.26 The experimental details are described in the Methods. Following the synthesis, we purified the particles using silica column chromatography and then characterized them using high-resolution transmission electron microscopy (HR-TEM). We have also used dynamic light scattering (DLS) to determine the size in solution. Freshly prepared blue fluorescent CDs are ∼6 nm in diameter, as shown in the HR-TEM image in Figure 1A.
Figure 1.
(A) TEM image of freshly prepared blue fluorescent CDs. Inset: HR-TEM image of a single particle. (B) TEM image of freshly prepared red fluorescent CDs. Inset: HR-TEM image of a single particle. (C) TEM image of freshly prepared acid-functionalized magnetic nanoparticles. (D) TEM image of multifunctional magneto-CD nanoparticles. Insets: EDX elemental mapping of Fe, C, and O in magneto-CD nanoparticles. (E) Emission spectra from blue fluorescent magneto-CD-attached nanoparticles at 380 nm excitation. (F) Emission spectra from a mixture of multicolor multifunctional CD-based nanosystems at 380 nm excitation. Blue fluorescent magneto-CD nanoparticles (110 ppm) and red fluorescent magneto-CD nanoparticles (180 ppm) were used for the fluorescence measurement. (G,H) Photograph of multicolor multifunctional CD-based nanosystems in the presence of UV light.
2.2. Synthesis and Characterization of Blue/Red Fluorescent Magneto-CD Nanoparticles
For the development of multicolor multifunctional CD-based nanosystems, initially we synthesized carboxylic acid-functionalized magnetic nanoparticles using coprecipitation method from ferric chloride and 1,6-hexanedioic acid, as we reported before15,16,30 and as shown in Schemes 1 and 2. The black Fe3O4 nanoparticle precipitate was separated from the supernatant using a neodymium magnet and thoroughly washed several times with water. Fe3O4 nanoparticles were characterized using HR-TEM and DLS.
Scheme 1. (A) Synthetic Route for the Development of Blue Fluorescent Carbon Dot-Attached Magnetic Nanoparticle-Based Hybrid Nanomaterials. (B) Synthetic Route for the Development of Anti-Salmonella-Antibody-Attached Blue Fluorescent Hybrid Nanomaterials.
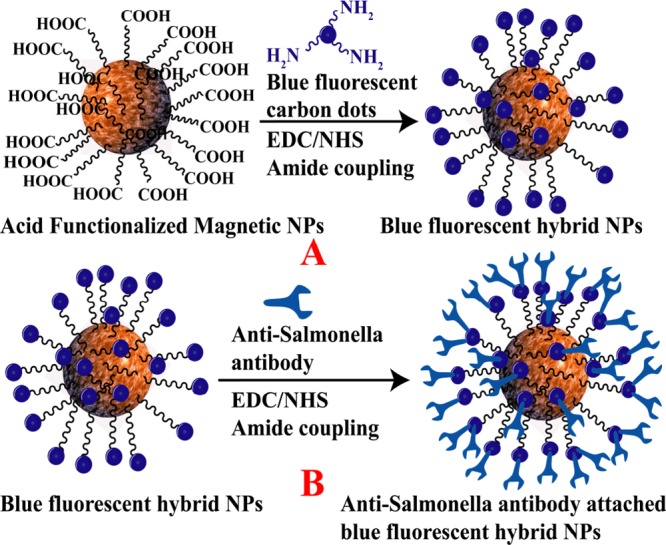
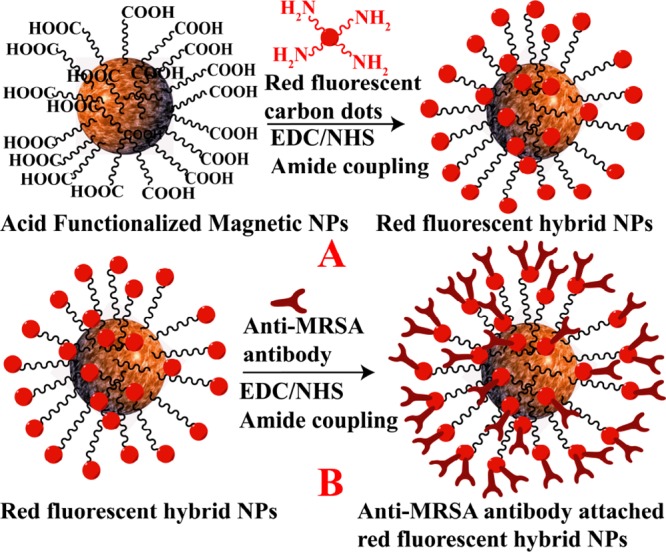
Scheme 2. (A) Synthetic Route for the Development of Red Fluorescent Carbon Dot-Attached Magnetic Nanoparticle-Based Hybrid Nanomaterials. (B) Synthetic Route for the Development of Anti-MRSA-Antibody-Attached Red Fluorescent Hybrid Nanomaterials.
The resultant HR-TEM image is shown in Figure 1C; particles have an average size of about ∼40 nm. DLS results reported in Table 1 also indicate that an average size is of 40 ± 5 nm for magnetic nanoparticles. Superparamagnetic properties were measured using a SQUID magnetometer, which indicates a specific saturation magnetization of 41.8 emu g–1 for the magnetite nanoparticles we developed. In the next step, 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide (EDC)-mediated esterification was used to produce magnetic nanoparticles attached with blue-red fluorescent CDs, as we have reported before.15 The final product, amide-coupled blue/red fluorescent CD–Fe3O4 nanoparticles, was separated using a magnet and washed several times with water to remove the excess CDs. HR-TEM, elemental mapping by energy-dispersive X-ray (EDX) spectroscopy, elemental mapping, and DLS results are reported in Figure 1 and Tables 1 and 2. Figure 1D shows the HR-TEM image for ∼55 nm-diameter purified blue fluorescent magneto-CD nanoparticles. It is noteworthy to observe that multifunctional CD-based nanosystems are about 15 nm larger than the unreacted magnetic nanoparticles. The EDX data shown in Figure 1D indicate the presence of iron, carbon, and oxygen in multifunctional CD-based nanosystems. The DLS data reported in Table 1 confirm a size of about 55 nm for blue fluorescent multifunctional CD-based nanosystems and 50 nm for red fluorescent magneto-CD nanoparticles. We determined superparamagnetic properties using the SQUID magnetometer, which indicate a specific saturation magnetization of 38.8 emu g–1 for the red magneto-CD nanoparticles and 36.2 emu g–1 for the blue magneto-CD nanoparticles.
Table 1. Size Distribution for Blue Fluorescent CDs, Acid-Functionalized Magnetic Nanoparticles, and Multifunctional CD-Based Nanosytems.
| nanoparticle description | size measured by DLS (nm) | size measured by SEM (nm) |
|---|---|---|
| blue fluorescent CDs | 6 ± 3 | 6 ± 3 |
| acid-functionalized magnetic nanoparticles | 40 ± 7 | 40 ± 5 |
| blue fluorescent magneto-CD nanoparticles | 55 ± 10 | 55 ± 8 |
Table 2. Size Distribution for Red Fluorescent CDs, Acid-Functionalized Magnetic Nanoparticles, and Magneto-Red Fluorescent CD Nanoparticles.
| nanoparticle description | size measured by DLS (nm) | size measured by SEM (nm) |
|---|---|---|
| red fluorescent CDs | 4 ± 2 | 4 ± 2 |
| acid-functionalized magnetic nanoparticles | 40 ± 7 | 40 ± 5 |
| red fluorescent magneto-CD nanoparticles | 50 ± 8 | 50 ± 8 |
Figure 1G,H shows the emission spectra from multicolor multifunctional CD-based nanosystems at 380 nm excitation. Experimental data indicate λmax of emission for blue fluorescent magneto-CD nanoparticles of around 462 nm and around 630 nm for red fluorescent magneto-CD nanoparticles. We used quinine sulfate as a standard (QY 54%) for the measurement of photoluminescence quantum yield (QY) for magneto-fluorescent CDs using our reported method.13−16Equation 1 was used to calculate the QY for blue fluorescent magneto-CD nanoparticles14−26
| 1 |
where the blue fluorescent magneto-CD nanoparticle is termed “mcd” and the quinine sulfate, which was used as the standard, is termed “ref”. The photoluminescence QY is denoted as Φ, and the absorbance, fluorescence intensity, and refractive index are denoted as A, I, and η, respectively. Using experimental results and eq 1, the QYs for blue fluorescent CD and blue fluorescent magneto-CD nanoparticles were determined to be 0.51 and 0.28 at 380 nm excitation. Similarly, the QYs for red fluorescent CD and red fluorescent magneto-CD nanoparticles were determined to be 0.24 and 0.12 at 380 nm excitation.
Figure 1G shows that the multicolor multifunctional CD-based nanosystems exhibit blue and red fluorescence, when excited at 380 nm UV light. Consequently, selective and simultaneous targeted imaging of drug-resistant MRSA and Salmonella DT104 is possible using magneto-CD nanosystems.
2.3. Developing Antibody-Conjugated Blue-Red Fluorescent Magneto-CD Nanoparticles
For selective binding, separation, and luminescence imaging of Salmonella DT104, we have developed anti-Salmonella-antibody-attached blue fluorescent magneto-CD nanoparticles. To accomplish this, we have developed amine-conjugated polyethylene glycol (NH2–PEG)-attached blue fluorescent magneto-CD nanoparticles and then Salmonella DT104-targeted antibody was attached with blue fluorescent magneto-CD nanoparticles, utilizing our reported method.14,15,30 As shown in Scheme 1B, we have used EDC/N-hydroxysuccinimide (NHS) chemistry for the formation of covalent bonds between the carboxyl groups of PEG and the primary amine groups of anti-MRSA antibody. Similarly, for selective binding and imaging of MRSA, we have developed anti-MRSA-antibody-attached red fluorescent magneto-CD nanoparticles using the above procedure. In this case, we have first developed PEG-coated red fluorescent magneto-CD nanoparticles and anti-MRSA antibody was then attached to the hybrid nanoparticle via EDC/NHS chemistry, as shown in Scheme 2B.
2.4. Determining the Possible Nanotoxicity and Photostability
To determine the possible nanotoxicity from our synthesized nanoprobes, we have determined the biocompatibility of the antibody-attached blue fluorescent magneto-CD nanoparticles. For this purpose, around 18 000 CFU/mL Salmolella DT104, MRSA, and Escherichia coli bacteria were incubated separately with antibody-attached blue fluorescent magneto-CD nanoparticles for 24 h. Similarly, 1.2 × 105 cells/mL normal skin HaCaT cells were incubated separately with antibody-attached multifunctional CD-based nanosystems for 24 h. In the next step, the number of live cells was counted using the colony-counting method37,39,40 for bacteria and MTT test14−16 for HaCaT cell lines. Figure 2A shows excellent biocompatibility for the multicolor multifunctional CD-based nanosystems, where more than 97% of bacteria or HaCaT cells are alive after one day of incubation.
Figure 2.
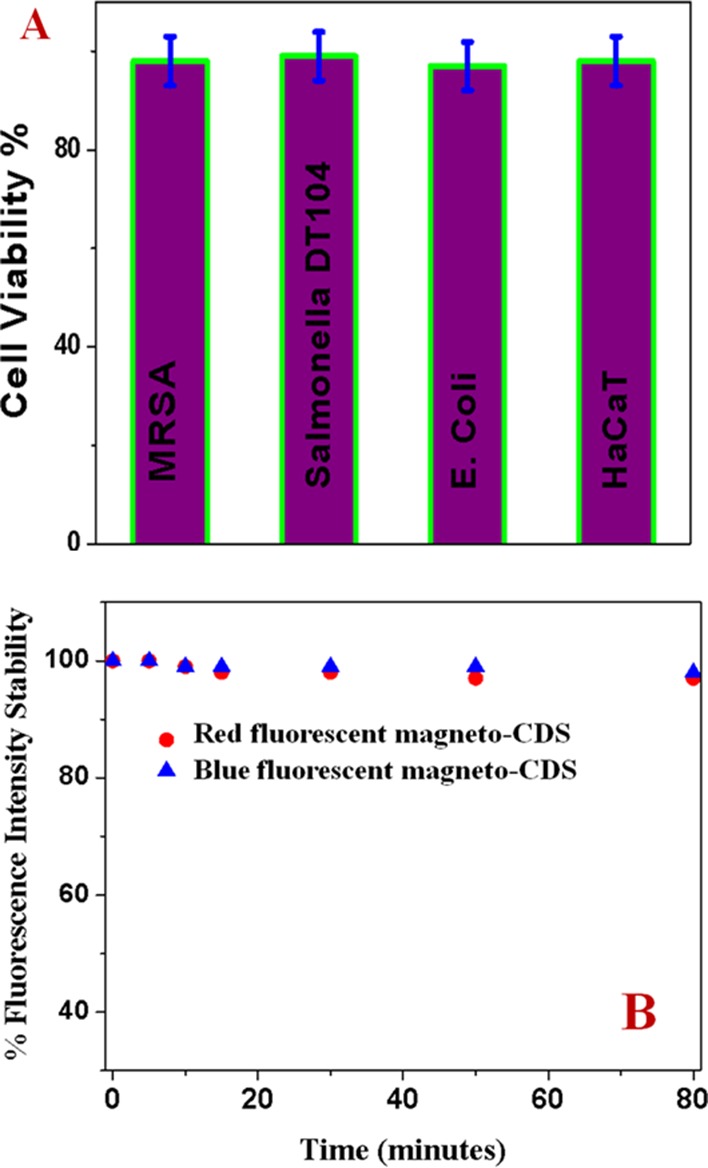
(A) Viability of cells treated with blue fluorescent CDs. (B) Fluorescence intensity for anti-MRSA-antibody-attached blue-red fluorescent CD nanoparticles as a function of time.
To determine the photostability of our synthesized multicolor multifunctional CD-based nanosystems, time-dependent luminescence experiments have been performed using 380 nm excitation for around 1.5 h exposure. Figure 2B indicates excellent photostability for red-blue fluorescent magneto-CD nanoparticles remain, where the luminescence intensity changed only less than 5% even more than 1 h of exposure to light.
2.5. Capturing and Identifying Salmonella DT104 and MRSA from Infected Blood
To determine whether multicolor multifunctional CD-based nanosystems could be used for capturing and imaging Salmonella DT104 and MRSA superbugs separately and together from the infected sample, 103 CFU/mL of superbugs were injected into 10 mL of citrated whole rabbit blood.
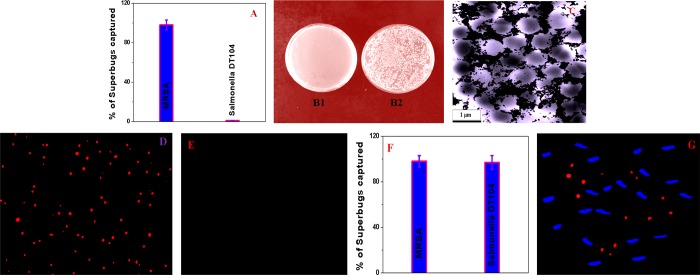
Then, 106 cells/mL peripheral blood mononuclear cells (PBMCs) were added into the mixture, which was gently shaken for over 90 min. The mixture was used as an infected blood sample for separation and imaging. Then, different concentrations of anti-MRSA-antibody-attached red fluorescent magneto-CD nanoparticles were added to the infected blood sample and mixed continuously for half an hour. In the next step, targeted MRSA-bound magneto-CD nanoparticles were separated from infected blood using a magnet. Finally, we used reverse transcription polymerase chain reaction (RT-PCR) technique, colony plating technique, and fluorescence mapping analysis15,30,37−39 to determine the percentage of superbugs that were captured, as reported in Figure 3. The RT-PCR data reported in Figure 3A indicate that around 100% of the MRSA was captured by the magneto-CD we developed. The colony-plating data reported in Figure 3B also indicate complete capture of superbugs. Figure 3C shows the TEM image, which indicates that magneto-CD nanoparticles are attached on the surface of MRSA. In Figure 3D, the red fluorescence image of MRSA superbugs indicates that bioconjugated magnetic-CD nanoparticles are capable of identifying MRSA. Figure 3E shows that the anti-MRSA-antibody-attached red fluorescent magneto-CD nanoparticles do not conjugate with different cells that are present in the infected blood. This lack of binding resulted in no luminescence image for the supernatant following magnetic separation. The above-reported data indicate that anti-MRSA-antibody-conjugated red fluorescent magneto-CD nanoparticles can be used to separate and identify MRSA superbugs from the infected sample.
Figure 3.
(A) Bar graph of MRSA removal efficiency using anti-MRSA-antibody-attached red fluorescent magneto-CD nanoparticles. (B1,B2) Agar plates of live MRSA bacteria: (B1) before and (B2) after magnetic separation. (C) TEM image of MRSA capture by anti-MRSA-antibody-attached blue fluorescent magneto-CD nanoparticles. (D) Luminescence image of captured MRSA superbugs by anti-MRSA-antibody-attached nanoprobes. (E) Luminescence image from supernatant after magnetic separation. (F) Bar graph of MRSA and Salmonella DT104 removal efficiency using both anti-MRSA-antibody-attached red fluorescent magneto-CD nanoparticles and anti-Salmonella blue fluorescent magneto-CD nanoparticles. (G) Luminescence image of MRSA and Salmonella superbugs after capture by the two nanoprobes.
To determine the selectivity for superbug capturing and identification of MRSA superbugs, the same experiment was performed using Salmonella DT104 superbug at different concentrations. As reported in Figure 3A, our experimental data indicate that anti-MRSA-antibody-conjugated red fluorescent magneto-CD nanoparticles are highly selective for MRSA. Thus, Salmonella DT104 superbug capture efficiency was less than 1%.
Next, to understand how versatile multicolor fluorescent magneto-CD nanoparticles are for simultaneous capturing of MRSA and Salmonella DT104 superbugs, experiments with spiked blood samples containing both MRSA and Salmonella DT104 were performed. In this case, 103 CFU/mL MRSA and 103 CFU/mL Salmonella DT104 were infected in the whole blood sample containing 1 million PBMCs. For separation and identification of both MRSA and Salmonella DT104 at the same time, anti-MRSA-antibody-attached red fluorescent magneto-CD nanoparticles and anti-Salmonella-antibody-attached blue fluorescent magneto-CD nanoparticles were added together in 10 mL of the infected blood sample. Figure 3F shows the RT-PCR data, which indicate that the multicolor multifunctional nanoprobes can be used for more than 98% separation of different superbugs from the infected sample. Figure 3G shows the multicolor luminescence image that reveals that anti-MRSA-antibody-attached red fluorescent magneto-CD nanoparticles and anti-Salmonella-antibody-attached blue fluorescent magneto-CD nanoparticles together can be used for the simultaneous identification of MRSA and Salmonella DT104. In the reported multicolor luminescence data, the red fluorescent superbug images are MRSA and blue fluorescent superbug images are Salmonella DT104. The reported data clearly support the premise that different antibodies-attached multicolor fluorescent magneto-CD nanoparticles can be used for capturing MRSA and Salmonella DT104 superbugs selectively and simultaneously from infected whole blood. Although we have shown that anti-Salmonella-antibody-conjugated blue fluorescent nanoparticles can be used for the separation and imaging of Salmonella DT104, because selectivity is highly dependent on anti-Salmonella antibody, it may not be able to separate Salmonella DT104 from a mixture of other closely related Salmonella strains such as Salmonella typhimurium. Similarly, anti-MRSA-antibody-conjugated red fluorescent nanoparticles may not be able to separate MRSA from the mixture of other closely related Staphylococcus strains such as Staphylococcus aureus.
2.6. Targeted Capturing and Killing of Salmonella DT104 and MRSA
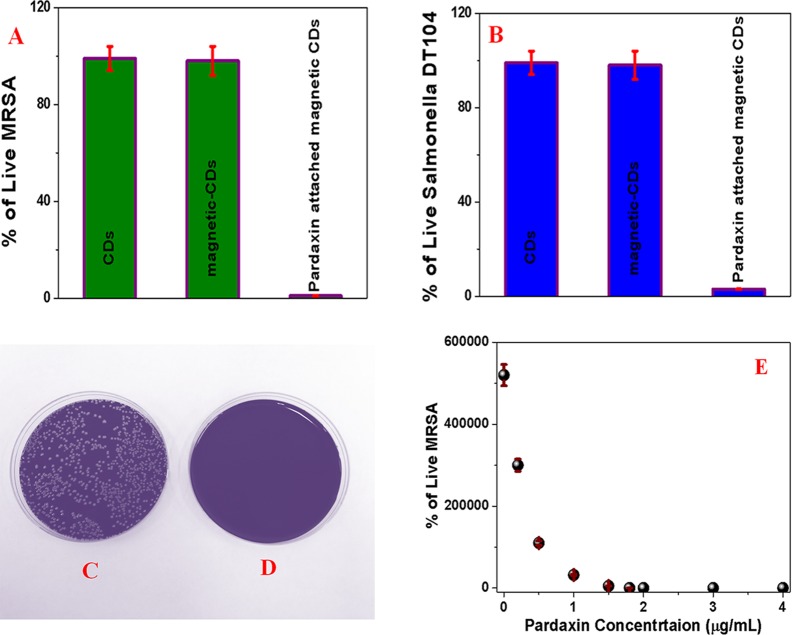
Because MRSA and Salmonella DT104 superbugs are resistant to several antibiotics, pardaxin antimicrobial peptide-conjugated anti-MRSA-antibody-attached red fluorescent magneto-CD nanoparticles and pardaxin antimicrobial peptide-conjugated anti-Salmonella-antibody-attached blue fluorescent magneto-CD nanoparticles were developed. For this purpose, amine groups of pardaxin antimicrobial peptides were attached to the acid-functionalized magnetic nanoparticle using EDC/NHS cross-linking via amide linkage. To determine whether the superbugs captured by antimicrobial peptide-conjugated fluorescent magneto-CD nanoparticles are alive or dead, magnetically separated particles were washed with water and then colony-counting technique was used to determine the percentage of live superbugs. Sample experiments in the absence of antimicrobial peptide conjugation were performed. As shown in Figure 4A–D, almost 100% of MRSA and Salmonella were killed when pardaxin antimicrobial peptide-conjugated fluorescent magneto-CD nanoparticles were used.
Figure 4.
(A) Percentage of live MRSA, when superbugs are captured by (i) anti-MRSA-antibody-attached fluorescent CD nanoparticles, (ii) anti-PSMA-antibody-attached fluorescent magneto-CD nanoparticles, and (iii) pardaxin-conjugated anti-PSMA-antibody-attached fluorescent magneto-CD nanoparticles. (B) Percentage of colonies of Salmonella DT104 compared with the control after capture by (i) anti-Salmonella-antibody-attached fluorescent CD nanoparticles, (ii) anti-Salmonella-antibody-attached fluorescent magneto-CD nanoparticles, and (iii) pardaxin-conjugated anti-Salmonella-antibody-attached fluorescent magneto-CD nanoparticles. (C,D) Agar plates of superbugs incubated (C) after being captured by a mixture of anti-MRSA-antibody-attached red fluorescent magneto-CD nanoparticles and anti-Salmonella-antibody-attached blue fluorescent magneto-CD nanoparticles and (D) after being captured by a mixture of pardaxin antimicrobial peptides-conjugated anti-MRSA-antibody-attached red fluorescent magneto-CD nanoparticles and pardaxin antimicrobial peptide-conjugated anti-Salmonella-antibody-attached blue fluorescent magneto-CD nanoparticles. (E) Plot shows how the percentage of live MRSA varies with the dosage level of pardaxin antimicrobial peptides.
On the other hand, more than 98% of superbugs were alive when pardaxin antimicrobial peptides are not conjugated with fluorescent magneto-CD nanoparticles. The observed very high killing efficiency of pardaxin antimicrobial peptide-conjugated fluorescent magneto-CD nanoparticles can be due to several facts. It is now well-documented that pore-forming peptides such as pardaxin exhibit great cytotoxicity against Gram-positive and Gram-negative bacteria.12,13 It is also reported that pardaxin is capable of elevating caspase-3/7 activities and disruption of the mitochondrial membrane potential.12,13 Pardaxin is also capable for accumulation of reactive oxygen species production.12,13 All above-described mechanisms are responsible for the death of superbugs in the presence of pardaxin antimicrobial peptide-conjugated fluorescent magneto-CDs nanoparticles. To understand the limit of dosage of the antimicrobial peptide to eradicate MRSA and Salmonella DT104 superbugs, we performed pardaxin concentration-dependent study, as reported in Figure 4E, which indicates that 1.8 μg/mL pardaxin is necessary to kill 100% of MRSA. A similar experiment was performed for Salmonella DT104, and we found that 2.4 μg/mL pardaxin is necessary to kill 100% of Salmonella DT.
3. Conclusions
In conclusion, this article reports the development of multicolor fluorescent CD-conjugated magnetic nanoparticles that are capable of selective separation and accurate identification of superbugs from infected blood. The new means of capturing and identifying MRSA and Salmonella DT104 superbugs from clinically relevant samples is clearly demonstrated. The reported data show that multicolor multifunctional CD-based nanosystems are capable of isolating MRSA and Salmonella DT104 superbugs from whole blood samples, followed by accurate identification via multicolor fluorescence imaging. Although we have shown that anti-Salmonella-antibody-conjugated blue fluorescent nanoparticles can be used for Salmonella DT104 imaging and anti-MRSA-antibody-conjugated red fluorescent nanoparticles can be used for MRSA imaging, we can use either blue or red fluorescent nanoparticles to image MRSA/Salmonella or other superbugs, just by changing the specific recognition unit such as antibody. Because multidrug-resistant superbugs are resistant to the antibiotics available in the market, the design of pardaxin antimicrobial peptide-attached multicolor fluorescent magneto-CDs for effective separation, accurate identification, and complete disinfection of MDR superbugs from infected blood is also reported. The reported data indicate that by combining pardaxin antimicrobial peptides with fluorescent magneto-CD nanoparticles, one can develop a novel multifunctional material that has great potential to be used for the identification of MDRB infection in clinics.
4. Methods
All chemicals including meta-phenylenediamine, para-phenylenediamine, ethanol, CH2Cl2 and MeOH, NaBH4, NH2–PEG, FeCl3·6H2O, and 1,6-hexanedioic acid were purchased from Fisher Scientific and Sigma-Aldrich. Superbugs, such as multidrug-resistant Salmonella DT104 and MRSA, and growth media were purchased from the American Type Culture Collection (ATCC, Rockville, MD).
4.1. Development of Blue Fluorescent Carbon Dots Using meta-Phenylenediamine
Blue fluorescent CDs were synthesized by hydrothermal heating of meta-phenylenediamine in ethanol using a reported method,26 as shown in Scheme 1. In brief, 0.9 g of meta-phenylenediamine was dissolved in 90 mL of ethanol. Then, the mixture was heated in an autoclave at 180 °C for 12 h. After that, the gray-colored blue fluorescent CDs were then further purified using silica column chromatography using CH2Cl2 and MeOH (1:1) as eluants. Yield: 0.09 g, 10%.
4.2. Development of Red Fluorescent Carbon Dots Using para-Phenylenediamine
The red fluorescent CDs were synthesized by microwave-assisted heating of para-phenylenediamine using a reported method,26 as shown in Scheme 2. In brief, 0.027 g of para-phenylenediamine was dissolved in 50 mL of ethanol, and the mixture was heated for 60 min using a domestic microwave oven (900 W). During this process, a mixture of ethanol and water (1:1) was added into the flask continuously. After that, the resultant solution was dried by solvent evaporation and further purified by silica column chromatography using a mixture of ethanol and ethyl acetate (1:1) as eluants. Yield: 0.014 g, 50%.
4.3. Design of Carboxylic Acid-Conjugated Fe3O4 Nanoparticles
We designed carboxylic acid-functionalized Fe3O4 magnetic nanoparticles using the co-precipitation method using ferric chloride and 1,6-hexanedioic acid, as we reported before15,16,30 and as shown in Scheme 1. details were reported before.15,16,30 At the end, Fe3O4 nanoparticles were separated from the supernatant using a magnet.
4.4. Design of Blue-Red Fluorescent Magneto-CD Nanoparticles
For the covalent attachment of blue-red fluorescent magneto-CDs with acid-functionalized magnetic nanoparticles, EDC-mediated esterification was used, as we reported before.15 The final product, amide-coupled blue-red fluorescent CD–Fe3O4 nanoparticles, was separated using a magnet and washed several times with water to remove the excess CDs.
4.5. Development of Antibody-Conjugated Fluorescent Magnetic Nanoprobes
For selective binding, separation, and luminescence imaging of Salmonella DT104, we developed an anti-Salmonella-antibody-attached blue fluorescent magneto-CD nanoparticles. To accomplish this, we developed amine-conjugated polyethylene glycol (NH2–PEG)-attached blue fluorescent magneto-CD nanoparticles, and then Salmonella DT104-targeted antibody was attached with blue fluorescent magneto-CD nanoparticles, utilizing our reported method.14,15,30 As shown in Scheme 1B, we used EDC/NHS chemistry for the formation of covalent bonds between carboxyl groups of PEG and primary amine groups of anti-MRSA antibody. Similarly, for selective binding and imaging of MRSA, we developed anti-MRSA-antibody-attached red fluorescent magneto-CD nanoparticles using the above-mentioned procedure. In this case, we first developed PEG-coated red fluorescent magneto-CD nanoparticles, and then anti-MRSA antibody was attached to the hybrid nanoparticle via EDC/NHS chemistry, as shown in Scheme 2B.
4.6. Development of Pardaxin Antimicrobial Peptide-Conjugated Hybrid Nanomaterials
Because MRSA and Salmonella DT104 superbugs are resistant to several antibiotics, pardaxin antimicrobial peptide-conjugated anti-MRSA-antibody-attached red fluorescent magneto-CD nanoparticles and pardaxin antimicrobial peptide-conjugated anti-Salmonella-antibody-attached blue fluorescent magneto-CD nanoparticles were developed. For this purpose, amine groups of pardaxin antimicrobial peptides were attached to the acid-functionalized magnetic nanoparticle using EDC/NHS cross-linking via amide linkages.
4.7. Superbug Sample Preparation
Salmonella DT104 and MRSA were purchased from the ATCC and then cultured according to the ATCC protocol, as we reported before.37,39,40 For the preparation of infected blood sample, different concentrations of Salmonella DT104 and MRSA were prepared by diluting the stock solution.
4.8. Multicolor Luminescence Imaging of Captured Superbugs
For the fluorescence imaging of Salmonella DT104 and MRSA superbugs, we used an Olympus IX71 inverted confocal fluorescence microscope fitted with a SPOT Insight digital camera, as we reported before.14−16,30
4.9. Finding the Percentage of Live Superbugs
After being magnetically captured, Salmonella DT104 and MRSA superbugs were transferred to colony-countable plates and incubated for 1 day. The percentage of live superbugs was determined using a colony counter (Bantex, model 920 A).
Acknowledgments
Dr. Ray thanks NSF-PREM grant # DMR-1205194, NSF CREST grant # 1547754, NSF RISE grant # 1547836, and NIH RCMI grant (# G12RR013459-13) for generous funding.
The authors declare no competing financial interest.
References
- World Health Organization . Antimicrobial Resistance: Global Report on Surveillance 2014; WHO, 2014.
- http://www.who.int/mediacentre/factsheets/fs194/en/ (accessed Dec 8, 2016).
- https://www.cdc.gov/drugresistance/ (accessed Dec 8, 2016).
- Wang L.-S.; Gupta A.; Rotello V. M. Nanomaterials for the Treatment of Bacterial Biofilms. ACS Infect. Dis. 2016, 2, 3–4. 10.1021/acsinfecdis.5b00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam S. J.; O’Brien-Simpson N. M.; Pantarat N.; Sulistio A.; Wong E. H. H.; Chen Y.-Y.; Lenzo J. C.; Holden J. A.; Blencowe A.; Reynolds E. C.; Qiao G. G. Combating multidrug-resistant Gram-negative bacteria with structurally nanoengineered antimicrobial peptide polymers. Nat. Microbiol. 2016, 1, 16162. 10.1038/nmicrobiol.2016.162. [DOI] [PubMed] [Google Scholar]
- Li X.; Robinson S. M.; Gupta A.; Saha K.; Jiang Z.; Moyano D. F.; Sahar A.; Riley M. A.; Rotello V. M. Functional Gold Nanoparticles as Potent Antimicrobial Agents Against Multi-Drug-Resistant Bacteria. ACS Nano 2014, 8, 10682–10686. 10.1021/nn5042625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X.; Chen X.; Yang J.; Jia H.-R.; Li Y.-H.; Chen Z.; Wu F.-G. Quaternized Silicon Nanoparticles with Polarity-Sensitive Fluorescence for Selectively Imaging and Killing Gram-Positive Bacteria. Adv. Funct. Mater. 2016, 26, 5958–5970. 10.1002/adfm.201602185. [DOI] [Google Scholar]
- Bodelón G.; Montes-García V.; López-Puente V.; Hill E. H.; Hamon C.; Sanz-Ortiz M. N.; Rodal-Cedeira S.; Costas C.; Celiksoy S.; Pérez-Juste I.; Scarabelli L.; La Porta A.; Pérez-Juste J.; Pastoriza-Santos I.; Liz-Marzán L. M. Detection and Imaging of Quorum Sensing in Pseudomonas aeruginosa Biofilm Communities by Surface-Enhanced Resonance Raman Scattering. Nat. Mater. 2016, 15, 1203–1211. 10.1038/nmat4720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilchie A. L.; Wuerth K.; Hancock R. E. W. Immune modulation by multifaceted cationic host defense (antimicrobial) peptides. Nat. Chem. Biol. 2013, 9, 761–768. 10.1038/nchembio.1393. [DOI] [PubMed] [Google Scholar]
- Wang F.; Fang R. H.; Luk B. T.; Hu C.-M. J.; Thamphiwatana S.; Dehaini D.; Angsantikul P.; Kroll A. V.; Pang Z.; Gao W.; Lu W.; Zhang L. Nanoparticle-Based Antivirulence Vaccine for the Management of Methicillin-Resistant Staphylococcus aureus Skin Infection. Adv. Funct. Mater. 2016, 26, 1628–1635. 10.1002/adfm.201505231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S. A.; Singh A. K.; Senapati D.; Fan Z.; Ray P. C. Nanomaterials for targeted detection and photothermal killing of bacteria. Chem. Soc. Rev. 2012, 41, 3193–3209. 10.1039/c2cs15340h. [DOI] [PubMed] [Google Scholar]
- Huang T.-C.; Lee J.-F.; Chen J.-Y. Pardaxin, an antimicrobial peptide, triggers caspase-dependent and ROS-mediated apoptosis in HT-1080 cells. Mar. Drugs 2011, 9, 1995–2009. 10.3390/md9101995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H.-N.; Pan C.-Y.; Chan Y.-L.; Chen J.-Y.; Wu C.-J. Use of the antimicrobial peptide pardaxin (GE33) to protect against methicillin-resistant Staphylococcus aureus infection in mice with skin injuries. Antimicrob. Agents Chemother. 2014, 58, 1538–1545. 10.1128/aac.02427-13. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Nellore B. P. V.; Kanchanapally R.; Pramanik A.; Sinha S. S.; Chavva S. R.; Hamme A.; Ray P. C. Aptamer-Conjugated Graphene Oxide Membranes for Highly Efficient Capture and Accurate Identification of Multiple Types of Circulating Tumor Cells. Bioconjugate Chem. 2015, 26, 235–242. 10.1021/bc500503e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pramanik A.; Vangara A.; Nellore B. P. V.; Sinha S. S.; Chavva S. R.; Jones S.; Ray P. C. Development of Multifunctional Fluorescent-Magnetic Nanoprobes for Selective Capturing and Multicolor Imaging of Heterogeneous Circulating Tumor Cells. ACS Appl. Mater. Interfaces 2016, 8, 15076–15085. 10.1021/acsami.6b03262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Z.; Shelton M.; Singh A. K.; Senapati D.; Khan S. A.; Ray P. C. Multifunctional Plasmonic Shell–Magnetic Core Nanoparticles for Targeted Diagnostics, Isolation, and Photothermal Destruction of Tumor Cells. ACS Nano 2012, 6, 1065–1073. 10.1021/nn2045246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu F.; Huang Y.; Zhang G.; Zhao R.; Yang H.; Zhang D. Targeted Bioimaging and Photodynamic Therapy of Cancer Cells with an Activatable Red Fluorescent Bioprobe. Anal. Chem. 2014, 86, 7987–7995. 10.1021/ac502103t. [DOI] [PubMed] [Google Scholar]
- Ding H.; Yu S.-B.; Wei J.-S.; Xiong H.-M. Full-Color Light-Emitting Carbon Dots with a Surface-State-Controlled Luminescence Mechanism. ACS Nano 2016, 10, 484–491. 10.1021/acsnano.5b05406. [DOI] [PubMed] [Google Scholar]
- Meziani M. J.; Dong X.; Zhu L.; Jones L. P.; LeCroy G. E.; Yang F.; Wang S.; Wang P.; Zhao Y.; Yang L.; Tripp R. A.; Sun Y.-P. Visible-Light-Activated Bactericidal Functions of Carbon “Quantum” Dots. ACS Appl. Mater. Interfaces 2016, 8, 10761–10766. 10.1021/acsami.6b01765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu I.-C.; Yu J.; Ye F.; Rong Y.; Gallina M. E.; Fujimoto B. S.; Zhang Y.; Chan Y.-H.; Sun W.; Zhou X.-H.; Wu C.; Chiu D. T. Squaraine-Based Polymer Dots with Narrow, Bright Near-Infrared Fluorescence for Biological Applications. J. Am. Chem. Soc. 2015, 137, 173–178. 10.1021/ja5123045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H.-Y.; Wu P.-J.; Kuo S.-Y.; Chen C.-P.; Chang E.-H.; Wu C.-Y.; Chan Y.-H. Quinoxaline-Based Polymer Dots with Ultrabright Red to Near-Infrared Fluorescence for in Vivo Biological Imaging. J. Am. Chem. Soc. 2015, 137, 10420–10429. 10.1021/jacs.5b06710. [DOI] [PubMed] [Google Scholar]
- Sun Y.; Cao W.; Li S.; Jin S.; Hu K.; Hu L.; Huang Y.; Gao X.; Wu Y.; Liang X.-J. Ultrabright and multicolorful fluorescence of amphiphilic polyethyleneimine polymer dots for efficiently combined imaging and therapy. Sci. Rep. 2013, 3, 3036. 10.1038/srep03036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker S. N.; Baker G. A. Luminescent carbon nanodots: Emergent nanolights. Angew. Chem., Int. Ed. 2010, 49, 6726–6744. 10.1002/anie.200906623. [DOI] [PubMed] [Google Scholar]
- Bao L.; Liu C.; Zhang Z.-L.; Pang D.-W. Photoluminescence-Tunable Carbon Nanodots: Surface-State Energy-Gap Tuning. Adv. Mater. 2015, 27, 1663–1667. 10.1002/adma.201405070. [DOI] [PubMed] [Google Scholar]
- Pramanik A.; Fan Z.; Chavva S. R.; Sinha S. S.; Ray P. C. Highly Efficient and Excitation Tunable Two-Photon Luminescence Platform for Targeted Multi-Color MDRB Imaging Using Graphene Oxide. Sci. Rep. 2014, 4, 6090. 10.1038/srep06090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang K.; Sun S.; Zhang L.; Lu Y.; Wu A.; Cai C.; Lin H. Red, Green, and Blue Luminescence by Carbon Dots: Full-Color Emission Tuning and Multicolor Cellular Imaging. Angew. Chem., Int. Ed. 2015, 54, 5360–5363. 10.1002/anie.201501193. [DOI] [PubMed] [Google Scholar]
- Ye R.; Xiang C.; Lin J.; Peng Z.; Huang K.; Yan Z.; Cook N. P.; Samuel E. L. G.; Hwang C.-C.; Ruan G.; Ceriotti G.; Raji A.-R. O.; Marti A. A.; Tour J. M. Coal as an abundant source of graphene quantum dots. Nat. Commun. 2013, 4, 2943–2948. 10.1038/ncomms3943. [DOI] [PubMed] [Google Scholar]
- Liu J.; Wickramaratne N. P.; Qiao S. Z.; Jaroniec M. Molecular-Based Design and Emerging Applications of Nanoporous Carbon Spheres. Nat. Mater. 2015, 14, 763–774. 10.1038/nmat4317. [DOI] [PubMed] [Google Scholar]
- Hu S.; Trinchi A.; Atkin P.; Cole I. Tunable Photoluminescence Across the Entire Visible Spectrum from Carbon Dots Excited by White Light. Angew. Chem., Int. Ed. 2015, 54, 2970–2974. 10.1002/anie.201411004. [DOI] [PubMed] [Google Scholar]
- Shi Y.; Pramanik A.; Tchounwou C.; Pedraza F.; Crouch R. A.; Chavva S. R.; Vangara A.; Sinha S. S.; Jones S.; Sardar D.; Hawker C.; Ray P. C. Multifunctional Biocompatible Graphene Oxide Quantum Dots Decorated Magnetic Nanoplatform for Efficient Capture and Two-Photon Imaging of Rare Tumor Cells. ACS Appl. Mater. Interfaces 2015, 7, 10935–10943. 10.1021/acsami.5b02199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim S. Y.; Shen W.; Gao Z. Carbon Quantum Dots and Their Applications. Chem. Soc. Rev. 2015, 44, 362–381. 10.1039/C4CS00269E. [DOI] [PubMed] [Google Scholar]
- Fu M.; Ehrat F.; Wang Y.; Milowska K. Z.; Reckmeier C.; Rogach A. L.; Stolarczyk J. K.; Urban A. S.; Feldmann J. Carbon Dots: A Unique Fluorescent Cocktail of Polycyclic Aromatic Hydrocarbons. Nano Lett. 2015, 15, 6030–6035. 10.1021/acs.nanolett.5b02215. [DOI] [PubMed] [Google Scholar]
- Zhang X.; Chen X.; Yang J.; Jia H.-R.; Li Y.-H.; Chen Z.; Wu F.-G. Quaternized Silicon Nanoparticles with Polarity-Sensitive Fluorescence for Selectively Imaging and Killing Gram-Positive Bacteria. Adv. Funct. Mater. 2016, 26, 5958–5970. 10.1002/adfm.201602185. [DOI] [Google Scholar]
- Crawford S. E.; Andolina C. M.; Smith A. M.; Marbella L. E.; Johnston K. A.; Straney P. J.; Hartmann M. J.; Millstone J. E. Ligand-Mediated “Turn On,” High Quantum Yield Near-Infrared Emission in Small Gold Nanoparticles. J. Am. Chem. Soc. 2015, 137, 14423–14429. 10.1021/jacs.5b09408. [DOI] [PubMed] [Google Scholar]
- Wang F.; Fang R. H.; Luk B. T.; Hu C.-M. J.; Thamphiwatana S.; Dehaini D.; Angsantikul P.; Kroll A. V.; Pang Z.; Gao W.; Lu W.; Zhang L. Nanoparticle-Based Antivirulence Vaccine for the Management of Methicillin-Resistant Staphylococcus aureus Skin Infection. Adv. Funct. Mater. 2016, 26, 1628–1635. 10.1002/adfm.201505231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y.-Q.; Zhu B.; Li Y.; Leow W. R.; Goh R.; Ma B.; Fong E.; Tang M.; Chen X. A Synergistic Capture Strategy for Enhanced Detection and Elimination of Bacteria. Angew. Chem., Int. Ed. 2014, 53, 5837–5841. 10.1002/anie.201310135. [DOI] [PubMed] [Google Scholar]
- Jones S.; Sinha S. S.; Pramanik A.; Ray P. C. Three-dimensional (3D) plasmonic hot spots for label-free sensing and effective photothermal killing of multiple drug resistant superbugs. Nanoscale 2016, 8, 18301–18308. 10.1039/c6nr05888d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai X.; Fan Z.; Lu Y.; Ray P. C. Multifunctional Nanoplatforms for Targeted Multidrug-Resistant-Bacteria Theranostic Applications. ACS Appl. Mater. Interfaces 2013, 5, 11348–11354. 10.1021/am403567k. [DOI] [PubMed] [Google Scholar]
- Kanchanapally R.; Nellore B. P. V.; Sinha S. S.; Pedraza F.; Jones S. J.; Pramanik A.; Chavva S. R.; Tchounwou C.; Shi Y.; Vangara A.; Sardar D.; Ray P. C. Antimicrobial Peptide-Conjugated Graphene Oxide Membrane for Efficient Removal and Effective Killing of Multiple Drug Resistant Bacteria. RSC Adv. 2015, 5, 18881–18887. 10.1039/c5ra01321f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nellore B. P. V.; Kanchanapally R.; Pedraza F.; Sinha S. S.; Pramanik A.; Hamme A. T.; Arslan Z.; Sardar D.; Ray P. C. Bio-Conjugated Cnt-Bridged 3D Porous Graphene Oxide Membrane for Highly Efficient Disinfection of Pathogenic Bacteria and Removal of Toxic Metals from Water. ACS Appl. Mater. Interfaces 2015, 7, 19210–19218. 10.1021/acsami.5b05012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meziani M. J.; Dong X.; Zhu L.; Jones L. P.; LeCroy G. E.; Yang F.; Wang S.; Wang P.; Zhao Y.; Yang L.; Tripp R. A.; Sun Y.-P. Visible-Light-Activated Bactericidal Functions of Carbon “Quantum” Dots. ACS Appl. Mater. Interfaces 2016, 8, 10761–10766. 10.1021/acsami.6b01765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reguera J.; de Aberasturi D. J.; Winckelmans N.; Langer J.; Bals S.; Liz-Marzán L. M. Synthesis of Janus Plasmonic–Magnetic, Star–Sphere Nanoparticles, and Their Application in SERS Detection. Faraday Discuss. 2016, 191, 47–59. 10.1039/c6fd00012f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi D.; Sadat M. E.; Dunn A. W.; Mast D. B. Photo-Fluorescent and Magnetic Properties of Iron Oxide Nanoparticles for Biomedical Applications. Nanoscale 2015, 7, 8209–8232. 10.1039/c5nr01538c. [DOI] [PubMed] [Google Scholar]