Figure 3.
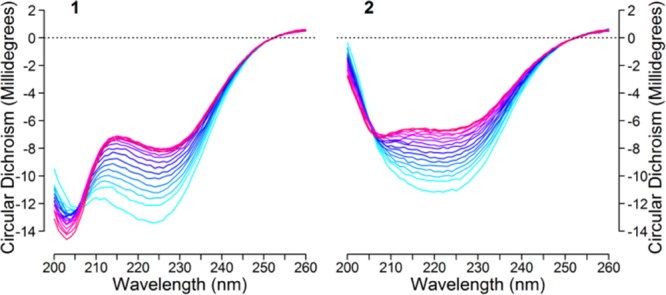
Concentration-corrected CD data obtained for 1 and 2 acquired in the 262–342 K interval for acetonitrile solutions (c ≈ 44 μM, b = 2 mm) indicate that both peptides adopt a twisted antiparallel β-hairpin conformation, with a strongly temperature-dependent negative band at 216–220 nm, permitting a detailed thermodynamic analysis of folding. The OH to CH3 substitution causes a significant change in the CD spectra.
