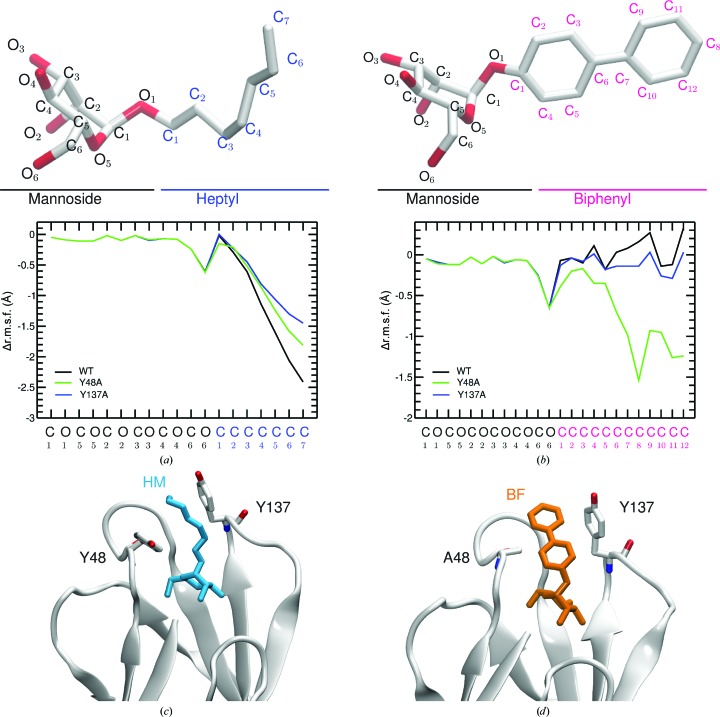
Figure 8.
Flexibility of ligands bound to WT FimH and the Y48A and Y137A mutants compared with the situation in water alone. The difference in flexibility for the WT (black), Y48A (green) and Y137A (blue) FimH MD trajectories (given as the Δr.m.s.f.) is plotted for the ligands (a) HM and (b) BF against the heavy-atom name of the ligand atoms. In addition, the ligand is depicted with atom names above each plot. The most representative conformation of (c) the HM ligand (cyan) and (d) the BF ligand (orange) is depicted for the trajectories with the lowest Δr.m.s.f. values (HM, WT; BF, Y48A). The protein is shown as a white cartoon; residues 48 and 137 are shown as ball-and-stick models and coloured atomwise.