Figure 1.
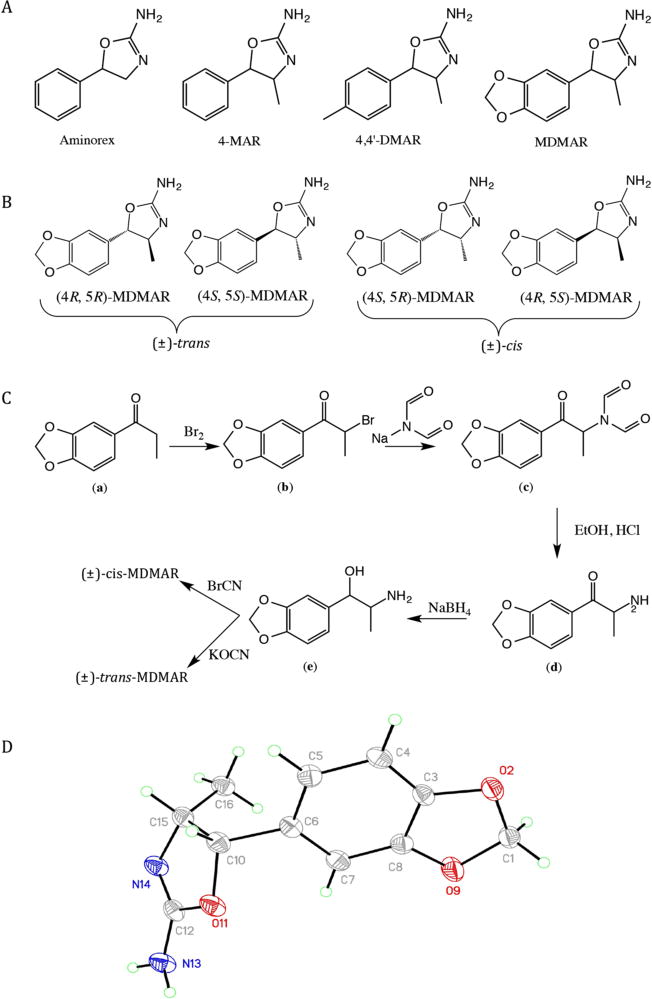
(A) Chemical structures of the three psychostimulants aminorex, 4-methylaminorex (4-MAR), para-methyl-4-methylaminorex (4,4′-DMAR) and the new psychoactive substance 3′,4′-methylenedioxy-4-methylaminorex (MDMAR). (B) Structural representation of all four MDMAR enantiomers. (C) Synthetic route to both (±)-cis- and (±)-trans- MDMAR. Both isomers were prepared from the same 3′,4′-methylenedioxynorephedrine prescursor (e) using cyanogen bromide or potassium cyanate to yield the (±)-cis and (±)-trans-MDMAR product, respectively. (D) Molecular structure of synthesized cis-MDMAR (50% displacement ellipsoids).
