Figure 7. Model of BRAF complex dynamics.
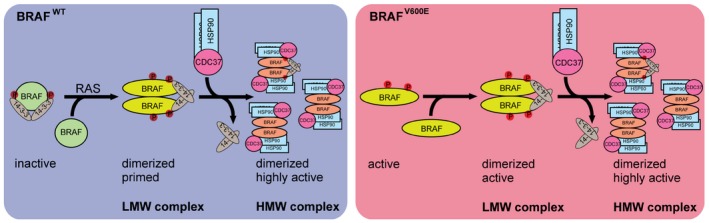
Left cartoon: BRAFWT is present in a monomeric form and depending on its phosphorylation status (S365, S729 marked in red) bound to 14‐3‐3. In the presence of active RAS, BRAF becomes phosphorylated and forms homo‐ or heterodimers by conformational changes in the kinase domain (Thevakumaran et al, 2015) and the concerted action of 14‐3‐3 proteins promoting dimerization at the RAF C‐termini. Dimerization promotes allosteric activation and a fully active conformation of BRAF, leading to recruitment of one asymmetric HSP90/CDC37 dimer per RAF protomer. Right cartoon: In contrast to BRAFWT, BRAFV600E is locked in an active conformation (Wan et al, 2004; Thevakumaran et al, 2015) and thus can remain active upon mutation of dimerization devices such as the C‐terminal 14‐3‐3 motif and the DIF (Röring et al, 2012). However, in cancer cells, BRAFV600E is predominantly found in a dimeric state. Upon translocation to the high molecular weight complexes BRAF variants become dephosphorylated reducing the interaction with 14‐3‐3 proteins and concurrently increasing the interaction with HSP90/CDC37. See Discussion for further details and additional references.
