FIG 9.
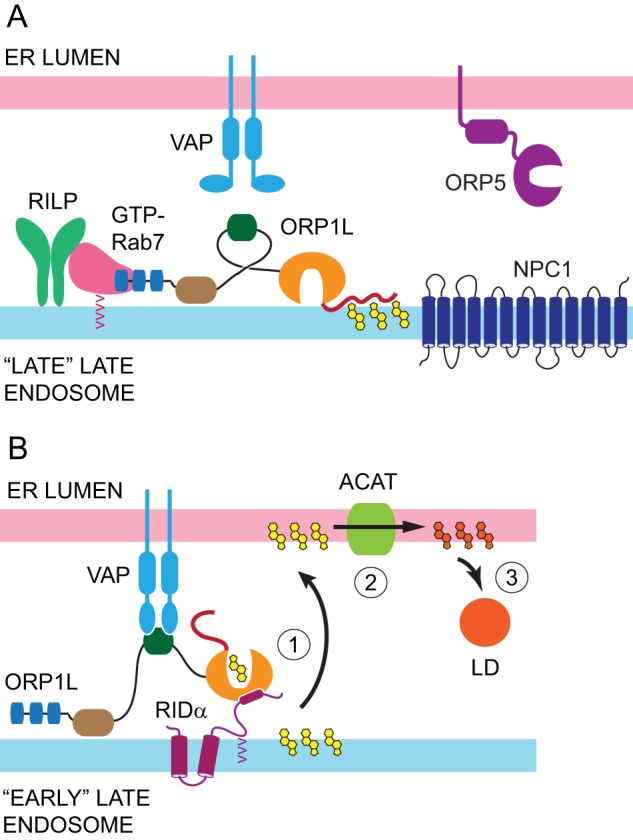
Ad reprograms ORP1L-VAP protein complexes to facilitate cholesterol transport in acutely infected cells. (A) ORP1L has been shown to form a tripartite complex with GTP-Rab7 and RILP that regulates late endosome motility of NPC1-containing “late-late endosomes.” The model depicts ORP1L conformation under high-cholesterol (yellow structures) conditions that tether the ORD-lid on endosomal membranes (34). This ORP1L conformation disrupts the interaction between ORP1L-FFAT and VAP and favors dynein motor activity downstream of RILP (34). (B) Our data support a model in which RIDα promotes cholesterol transport to ER (1) where ACAT catalyzes formation of CEs (orange structures) (2) stored in cytoplasmic LDs (3) via a direct interaction between RIDα-CT and the ORP1L-ORD in RIDα-induced endosome maturation intermediates (“early-late endosomes”). See Fig. 4B for a key to the ORP1L domain structure.
