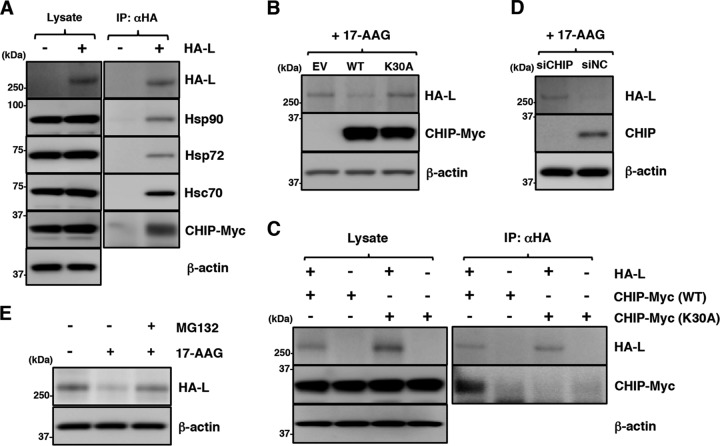
FIG 6.
Disruption of Hsp90 function induces degradation of the MuV L protein through a CHIP-mediated proteasomal pathway. (A) 293T cells were transfected with pcDNA-CHIP-Myc/His (WT) and/or pCAGGS-HA-MuV-L. At 24 h posttransfection, the cell lysates were subjected to immunoprecipitation with anti-HA antibody and to immunoblotting with the indicated antibodies. (B) 293T cells were cotransfected with pCAGGS-HA-MuV-L and pcDNA-CHIP-Myc/His (WT or K30A). At 24 h posttransfection, the cells were treated with 0.5 μM 17-AAG for 4 h, and then the cell lysates were subjected to immunoblotting with the indicated antibodies. EV, empty vector. (C) HA-MuV-L was coexpressed with CHIP-Myc (WT or K30A) in 293T cells, immunoprecipitated with anti-HA antibody, and immunoblotted with the indicated antibodies. (D) At 48 h posttransfection with either siCHIP or siNC, 293T cells were transfected with pCAGGS-HA-MuV-L. After 24 h, the cells were treated with 0.5 μM 17-AAG for 4 h, and then the cell lysates were subjected to immunoblotting with the indicated antibodies. (E) 293T cells were cotransfected with pCAGGS-HA-MuV-L and pcDNA-CHIP-Myc/His (WT). At 24 h posttransfection, the cells were treated with 0.5 μM 17-AAG and/or 10 μM MG-132 for 4 h, and then the cell lysates were subjected to immunoblotting with anti-HA and anti-β-actin antibodies.