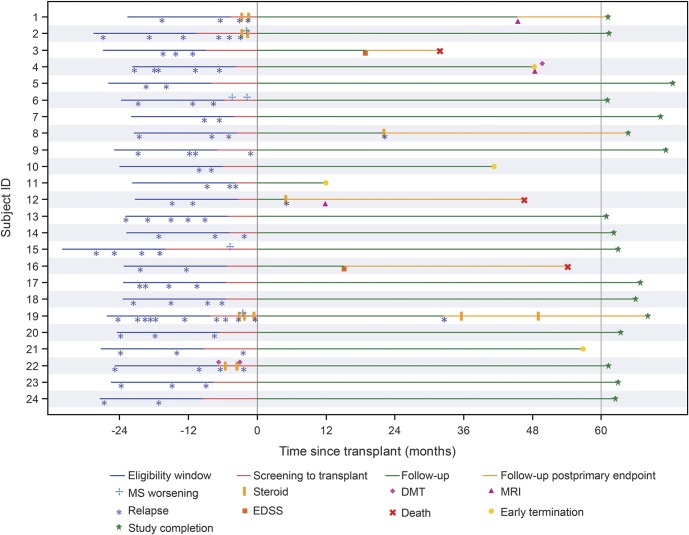
Figure 1. Patient-level pretransplant characteristics and outcomes.
Patient-level pretransplant characteristics and study outcomes are presented for the 24 transplanted participants. Time is measured in months relative to day 0, the day of graft infusion. The eligibility window is defined as the period of time beginning 18 months prior to the screening visit. At screening, the number of relapses, as defined in the protocol, that occurred during the eligibility window were identified retrospectively and documented in the clinical database, for the purpose of determining eligibility. The period of screening to transplant is defined as the time from determination of protocol eligibility until day 0. To obtain a more complete understanding of the events that occurred during the time from screening to day 0, we retrospectively investigated reports of multiple sclerosis (MS) attack, to determine if the participant had met the protocol definition of relapse, or if the event was a less severe MS worsening event. MS worsening is a new neurologic sign or symptom that does not meet criteria for relapse, and was documented only during the period from screening to day 0. Patients 2, 6, 14, 15, 19, and 22 experienced an Expanded Disability Status Scale (EDSS) increase of at least 0.5 during the period of screening to transplant. Study completion for some participants occurred beyond the 28-day visit window for the year 5 visit, due to scheduling difficulties. The participant flow diagram is presented as figure e-1 in the current publication. A total of 18 participants completed the year 4 assessment and 17 participants completed the year 5 assessment. MS disease activity is reduced for participants posttransplant as compared to prior to treatment. DMT = disease-modifying therapy.