Abstract
Brain vasculature acts in synergism with neurons to maintain brain function. This neurovascular coupling, or trophic coupling between cerebral endothelium and neuron, is now well-accepted as a marker for mapping brain activity. Neurovascular coupling is most active in the perivascular region, in which there are ample opportunities for cell-cell interactions within the neurovascular unit. This trophic coupling between cells maintains neurovascular function and cellular plasticity. Recent studies have revealed that even adult brains contain multiple stem cells of various lineages, which may provide cellular plasticity through the process of differentiation among these stem cell populations. In this chapter, we provide an overview of the process by which neurovascular components contribute to cellular plasticity in the cerebral perivascular regions, focusing on mechanisms of cell-cell interaction in adult brain.
Keywords: perivascular region, neurovascular coupling, neurogenesis, oligodendrogenesis, neurovascular niche, oligovascular niche, neurovascular unit
1. Introduction
Trophic coupling between different brain cells plays an essential role in maintaining normal brain function. In this regard, the concept of the neurovascular unit is a useful depiction of brain physiology and pathophysiology. The neurovascular unit provides a conceptual framework in which cell-cell interactions between neuronal, glial, and vascular elements constitute a functional entity 1–7. One important phenomenon within the neurovascular unit is that of neurovascular coupling, wherein neural activity evokes local blood flow changes. Imaging techniques including PET, fMRI, and fNIRS utilize the cerebrovascular-neuron interaction as a principle mechanism to map brain activity (see Chapters 4–6).
Neurovascular coupling takes place most prominently in the perivascular region (the microenvironment surrounding the microvasculature). This perivascular pocket may also provide an opportunity for trophic coupling between other types of cells. For example, the perivascular region gives rise to the neurovascular niche and gliovascular niche, wherein signaling pathways between cerebral endothelial cells and neuronal/glial precursor cells help mediate pockets of ongoing neurogenesis, gliogenesis and angiogenesis in adult brain 8,9. These intricate interactions are maintained during the remodeling phase after brain injury. Angiogenic signals are known to enhance neurogenesis after stroke 8,10. In turn, neuroblasts migrate along perivascular routes, and the process of neurogenesis enhances vascular re-growth 11. This cellular plasticity is an important mechanism in brain remodeling after injury.
Recent studies have revealed that even in the adult brain, mechanisms of cellular plasticity are retained to some extent. Adult brains possess stem cells from multiple lineages in the perivascular region, and coordination of self-renewal and differentiation in those stem cell populations maintains brain homeostasis. Notably, cell-cell trophic coupling may contribute to the cellular plasticity in the perivascular region, which plays an important role in repair responses in the adult brain after injury. In this chapter, we review mechanisms of cellular plasticity in the neurovascular unit, focusing on cell-cell interaction in the perivascular region.
2. Cellular plasticity and function of neurovascular unit components
The neurovascular unit is composed of neurons, glial cells (astrocytes, microglia, oligodendrocytes), and vascular cells (endothelial cells, pericytes). The dynamic interactions among these components regulate complex brain function, an example of which is that of adjustment of local blood flow by neural activity. Recent research advances have shown that these neurovascular unit components have diverse heterogeneity in their morphology, developmental origin, gene expression profile, physiological properties, function, and response to injury and disease 12–17. This diversity is a major contributor that underlies the cellular plasticity of neurovascular unit components in the adult brain.
Within the neurovascular unit, cell plasticity may come from differentiation of progenitor cells in the perivascular region. As noted, several kinds of progenitor cells reside even in the adult brain, and with trophic support by neighboring cells, they differentiate as needed for maintenance and/or remodeling of the relevant brain region. In this regard, neuronal stem/progenitor cells (NSPCs) play an essential role because they have the potential to differentiate and mature into all the subtypes of neurons and glial cells. They undergo both self-renewal and diversification into other types of cells; the former through symmetric proliferation and the later through asymmetric proliferation. The cellular plasticity exhibited by NSPCs during differentiation (i.e. neurogenesis and gliogenesis) is partly a response to intrinsic or extrinsic mechanisms, which trigger the pathways that determine the eventual cell fate. For example, NSPCs respond to inner or outer stimuli such as epigenetic regulation as in chromatin remodeling18, cellular signaling by cell-cell communication19 and the action of local trophic factors.
In this section, we briefly review the function of neurovascular components, focusing on cell differentiation mechanisms. Because mechanisms of adult neurogenesis and gliogenesis may mirror the cell differentiation steps observed during embryonic development, we also overview intrinsic and extrinsic signaling mechanisms for cell differentiation during brain development.
2.1. Neurons
Radial glia (RG), which is the progenitor cell for neural/glial cells, is morphologically characterized by the projection of its processes from the ventricular zone (VZ) to the meningeal zone with apico-basal polarity during the embryonic stage of brain development. During this period, RG switches its role among three main functions: self-proliferation, neurogenesis and gliogenesis (astrogenesis). NSPCs, which are originally derived from RG, generate both neurons and astrocytes even in cell culture conditions 20. Thus far, several intrinsic signaling pathways, such as Notch, Wnt and JAK/STAT signaling, have been found to have the ability to maintain NSPCs in undifferentiated states during the self-proliferation phase. In addition, chromatin remodeling of DNA by methylation and histone modification has also been shown to contribute to the plasticity of neurons and glial cells during the developmental stage (Fig. 1).
Figure 1.
Cellular signaling in neurogeneis and gliogenesis during brain development. Abbreviation: RG, radial glia; OPC, oligodendrocyte progenitor cell; GM, gray matter; WM, white matter; SVZ, subventricular zone.
Notch signaling is one of the major mediators of cell fate determination in cells of neural lineage. Notch signaling is mediated by the Notch receptors activated by ligand Jagged on the surface of the neighboring cells 21. The targets are transcriptional factors Hes (Hes1 and Hes5), which inhibit the neural differentiation of NSPC. The Notch-mediated cell-cell interactions spread to surrounding cells to maintain the undifferentiated conditions in their surrounding, which is known as ‘lateral inhibition’. Notch 1 knockout in mature neurons did not change the morphology but altered dendritic spines and disrupted long-term potentiation and long-term depression, leading to deficits in performing several memory tasks in mice 22.
Phase-dependent activation of Wnt signaling is another critical regulator of neuronal cell fate. Binding of Wnt to its receptor Frizzled inhibits the degradation of β-catenin, which binds the T-cell factor to upregulate downstream genes. The importance of β-catenin in neurogenesis is illustrated by the fact that β-catenin deletion is associated with impaired neurogenesis from immature neuron progenitor cells 23. Among genes downstream of Wnt signaling, Ngn1 and Ngn2 are key regulators that are highly expressed transcriptional factors during the neurogenesis phase 24,25. Ngn regulates neurogenesis and gliogenesis in a complex way. Ngn strongly promotes neurogenesis to constitute the six layers of brain cortex in an order from deep to superficial layers, and Ngn negatively regulates JAK-STAT-mediated demethylation of the GFAP promoter region and inhibits NSPC differentiation into astrocytes 26. Ngn2-knock out mice exhibit prominent decrements in neurogenesis and gliogenesis 27. Although Wnt signaling upregulates the expression of Ngn during the neurogenesis phase, its effects on chromatin remodeling by histone-modification of the promoter region of Ngn in the later phase results in decreased neurogenesis and Wnt-induced chromatin remodeling blunts the sensitivity to Wnt signaling, leading to a shift from neurogenesis to gliogenesis 28. Since precise mechanisms as to how Ngn regulates neurogenesis/gliogenesis are still mostly unknown, elucidating phase- and region-dependent mechanisms of Ngn signaling in NSPC function is warranted for future studies.
Taken together, phase-dependent cellular signaling and epigenetic modification in the promoter region of genes related to neurogenesis and gliogenesis are key regulators involved in the shift from neurogenesis to gliogenesis. Further studies will be needed to elucidate how these factors act synergistically to organize the process of neurogenesis by maintaining asymmetrical differentiation, and contribute to phase-dependent cellular plasticity.
2.2. Glial cells (astrocytes, oligodendrocytes, and microglia)
Astrocytes are mainly generated from three distinct areas in the brain: VZ, subventricular zone (SVZ) and the distributed localized area 29. RG in the VZ generates astrocytes in white matter during the gliogenesis phase. Glial progenitor cells in SVZ migrate to the cortical or subcortical layer of the brain and generate astrocytes in both gray and white matter. These newly generated astrocytes are morphologically categorized into two types - protoplasmic astrocytes in gray matter and fibroblastic astrocytes in white matter.
Several signaling pathways have been identified to modulate NSPC differentiation into astrocytes. As mentioned above, a central one is JAK/STAT signaling, in which JAK phosphorylates STAT1/3 and promotes the dimerization of STAT1/3 to up-regulate GFAP expression. Notch signaling is also an important regulator of GFAP gene expression both genetically and epigenetically. Notch intracellular domain (NICD)/CBF1 complex upregulates GFAP via direct binding to the GFAP promoter region. There are CpG methylated regions on the GFAP promoter to which Notch signaling-activated NFIA binds and prevents Dnmt1 from accessing the promoter region 30. This leads to demethylation of the promoter region and upregulation of GFAP expression.
Besides neurons and astrocytes, oligodendrocyte lineage cells are also generated from NSPCs. In general, oligodendrocytes are derived from so-called oligodendrocyte precursor cells (OPCs), which originate from NSPCs. However, there is ongoing debate as to whether oligodendrocytes should be generated from OPCs with fate-restricted lineage or not, since several studies have shown that OPCs retain context-dependent lineage plasticity in the differentiation period 31,32.
Historically, OPCs have been purified from rat optic nerves by immunopanning with antibody against A2B5 and identified as bi-potential oligodendrocyte-type2 astrocyte progenitor cells (O-2A cells) 33, which indicate that OPCs could differentiate into oligodendrocytes as well as astrocytes. In the postnatal period, OPCs appear in the SVZ and diffusely distributed to cover the entire parenchyma of the brain. Even in the adult CNS, OPCs have been found to comprise 2–8 % of all the cells 34,35. While majority of SVZ derived-OPCs can differentiate into oligodendrocytes, some may directly differentiate into protoplasmic astrocytes in gray matter. Actually, OPCs contribute to about 40 % of protoplasmic astrocytes in the gray matter of the ventral forebrain36. In addition, when purified OPCs were transplanted into the glia-depleted mice, they were differentiated into both oligodendrocytes and astrocytes 37. Moreover, when transplanted into myelin basic protein (MBP)-depleted conditions, some OPCs were differentiated into GFAP positive cells, while others expressed MBP, a marker of mature oligodendrocytes 38. Astrocyte generation from OPCs is limited during the initial postnatal period. These data suggest that OPCs are capable of differentiating into different types of cells, depending on the condition of the surrounding environment 39.
Thus far, several key mechanisms have been identified in cell fate decisions for oligodendrocyte lineage cells. Olig2 is a critical transcriptional regulator, which directs OPCs to become oligodendrocytes during embryogenesis 40–42. In fact, in Olig2-deficient conditions, OPCs were directed to become astrocytes, and postnatal hypomyelination was observed in the neocortex and corpus callosum 43. Epigenetic regulation such as DNA methylation and histone modification associated with chromatin remodeling was also shown to be involved in cell specification for oligodendrocytes 44. Treatment with HDAC inhibitor or genetic ablation of HDAC1/2 in OPCs resulted in failure of differentiation in vivo 45,46. Several groups have confirmed that HDAC activities are required for efficient myelination 47. For example, HDACs act in conjunction with the transcription factor YY1 to start myelin gene expression by suppressing Id4 and Tcf4 48. Neuronal activity 49, microRNAs 50, and Notch signaling 51 are also well-defined mechanisms for cell fate decisions in oligodendrocyte lineage cells. Working together, the factors and signaling pathways discussed here coordinately regulate oligodendrocyte generation during development.
Microglia, which originates from mesodermal yolk sac tissue during development 52,53, is a major cell type for the immune response in brain. After infiltrating into the brain during development, microglia reside and proliferate locally. In terms of cellular plasticity in the neurovascular unit, microglia plays a major role in synaptic pruning during development. The fractalkine receptor (CX3CR1), a marker of microglia 54, has been shown to be involved in the cytokine-mediated pathway in which synaptic plasticity would dynamically be formed by the interaction between neurons 55. Also, microglia responds to neurotransmitter released by neuronal activity and contributes to the synaptic pruning via fractalkine/fractalkine receptors (CX3CL1/CX3CR1) signaling56,57. Genetic fate mapping studies using CX3R1-knockout mice have revealed deficits in multiple learning abilities as well as a remarkable reduction in glutamatergic synapse formation 58,59. The function of CX3R1 is partially dependent on microglia-derived BDNF activity. The homeostasis of microglia is dependent on CSF-1/CD115 receptor because its inhibition results in complete depletion of brain microglia 60. These findings support the idea that in addition to immune responses, microglia play an important role in maintaining neurovascular homeostasis including cellular plasticity in neurons.
2.3. Vascular cells (cerebral endothelial cells and pericytes)
Vascular cells constitute a major cellular element of the brain, and cerebral endothelial cells play a central role in the neurovascular unit. The cerebrovascular system was traditionally seen as a passive conduit for blood, however, recent research has revealed that the microvasculature plays an active role in maintaining brain homeostasis. Cerebral endothelial cells are differentiated from endothelial progenitor cells (EPCs). EPCs originate from mesodermal cells, and are responsible for vasculogenesis such as the formation of capillary network in the developing embryo. In addition, EPCs also contribute to angiogenesis – in the setting of remodeling and repairing of the capillary network 61,62.
In addition to its role as a conduit for blood flow, cerebral endothelial cells act as a source of multiple mediators to regulate neurovascular plasticity. Through secreting soluble factors, cerebral endothelial cells control neuronal regeneration through the processes of proliferation, migration, and differentiation of NSPCs 63. Major mediators from cerebral endothelial cells are nitric oxide (NO)64,65, SXCL12 (SDF1), vascular endothelial growth factor (VEGF)66, brain-derived neurotrophic factor (BDNF)67 and pigmented epithelium-derived factor (PEDF). These endothelial-derived factors may act in concert with secreting factors from other neurovascular unit components to coordinate neurogenesis, neural plasticity and NSPC division during the developmental stage. For example, NO inhibits epidermal growth factor receptor (EGFR)-dependent PI3K/Akt signaling68 and acts as a tonic repressor of neurogenesis in vivo. VEGF plays multiple functions in maintaining the fenestration permeability of the vessel69, activity-induced neurogenesis70 and synaptic plasticity such as enhancement of the long-term potentiation response66. BDNF is reported to support the recruitment of newly generated neurons in SVZ71. PDGF acts to promote NSC renewal through potentiating Notch-dependent transcription72. Since cerebral endothelial cells play a central role in the neurovascular unit, future studies are needed to further investigate the mechanisms by which cerebral endothelial cells contribute to cellular plasticity within the neurovascular unit.
Within the neurovascular unit, pericytes play several unique roles. Pericytes are especially abundant in the brain vasculature. Similar to other neurovascular unit components, pericytes also interact with neighboring cells. For example, cerebral vasculature is covered with a 1:1–3 ratio between endothelial cells and pericytes 73–75, and pericytes are well known to regulate the integrity of blood-brain barrier 76,77. In addition, pericytes and endothelial cells reciprocally support each other in the perivascular area through the secretion of soluble factors. Platelet-derived growth factor-receptor β (PDGFRβ) is a constitutive marker of pericytes, and endothelial cells secrete PDGFβ to activate the recruitment and proliferation of pericytes. PDGF-β/PDGFRβ knockout mice suffer from a deficiency pericytes in the brain as well as other organs such as kidney, heart and lung 78. On the other hand, pericytes secrete Angiopoietin-1, the receptor for which (Tie-1), is predominantly expressed in endothelial cells and promotes endothelial maturation and stability 79,80. In addition to endothelium-pericyte interactions, recent studies demonstrated that in the perivascular region, pericytes may also exchange soluble factors with OPCs to support their function 81.
As discussed above, through secreting trophic factors, pericytes regulate cellular plasticity through their involvement in angiogenesis and oligodendrogenesis in the perivascular area. However, pericytes may contribute to cellular plasticity in a more direct way. Early studies have demonstrated that pericytes in the brain originate from mesenchyme cells that localize on the abluminal side of the endothelial cells 82. Recent studies in turn have stressed that the pericytes exhibit stem-cell-like properties, which enable them to differentiate into a variety of cell types, such as osteoblasts, adipocytes, chondrocytes, vascular smooth muscle and skeletal muscle. In fact, under ischemic conditions, pericytes can be reprogrammed into neurons 83. As illustrated by these examples, the pericyte is an important cell in the regulation of cell plasticity in the perivascular region.
3. Neurovascular/Oligovascular niche mediates neurogenesis and oligodendrogenesis in the neurovascular unit
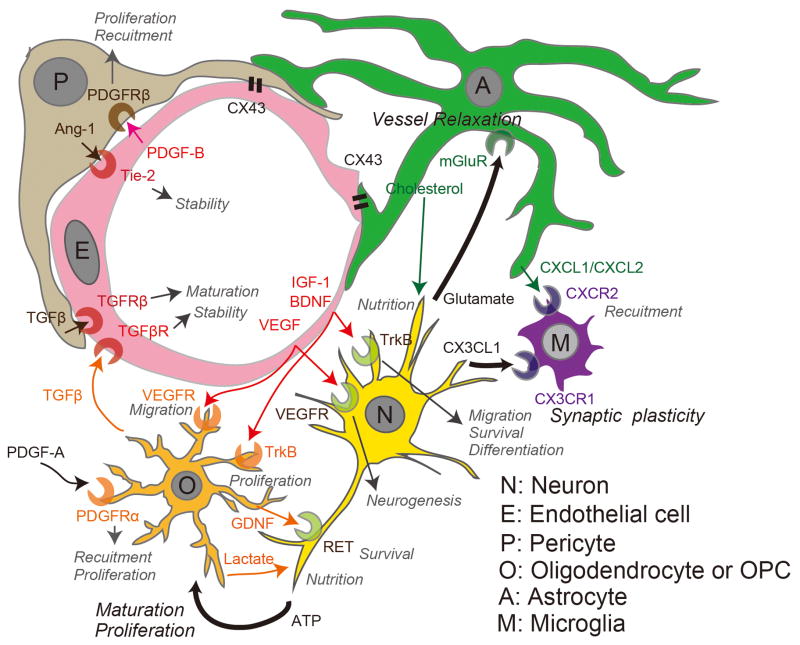
The components of the neurovascular unit coordinately affect cellular plasticity through self-renewal, cell fate, and synaptic plasticity, in processes that respond to stimulation by intrinsic and extrinsic factors (Figure 2). In this regard, cerebral endothelial cells play a central role in cellular plasticity because they produce the neurovascular and oligovascular niches to support neurogenesis and oligodendrogenesis.
Figure 2.
Interactive behavior in the neurovascular unit for systematic plasticity. Trophic factors are secreted from each neurovascular unit component to affect the function of its target cell. Abbreviation: A, Astrocyte; P, Pericyte; O, Oligodendrocyte or oligodendrocyte progenitor cell; N, Neuron; M, Microglia; E, Endothelial cell.
3.1. Neurovascular niche
As noted in the introduction section, cell-cell interaction in the neurovascular niche is one of the major facets within the neurovascular unit. Cerebral endothelial cells and NSPCs are closely related in the perivascular region in the processes of neurogenesis and angiogenesis 8. In this neurovascular niche, a number of trophic factors such as VEGF, BDNF, EGF and FGF2 are released from endothelial cells to upregulate NSPC expansion for neurogenesis. This is an important mechanism for adult neurogenesis in the adult brain. Additionally, after brain injury, cerebral endothelial cells respond to ischemic or traumatic stress by releasing necessary soluble factors that are important in the recovery of cellular functions.
In turn, NSPCs (and neurons) can regulate development of functional integrity in the neurovascular unit by secreting the factors such as VEGF that induce endothelial cell migration and guide vessel sprouting 84. For example, neuroblasts migrate along perivascular routes and the promotion of neurogenesis enhances vascular re-growth 11. Besides angiogenesis, neuronal activity also regulates blood flow in the local area of the brain (e.g. neurovascular coupling). This is mediated at least partly by the neuron-to-astrocyte signaling in which glutamate-mediated Ca2+ elevation in astrocytes promotes dilation of the arterioles 85 (see Chapter 2 by Nuriya and Hirase).
3.2. Oligovascular niche
Similar to the neurovascular niche, the oligovascular niche mediates interactions between cerebral endothelial cells and OPCs, mostly in cerebral white matter 86,87. OPCs are widely distributed even in adult brain 88–90 and when guided to the injured site, they contribute to myelin repair after brain injury 91. At the same time, cerebral endothelial cells may promote the survival and proliferation of perivascular OPCs 81 via FGF-2 and BDNF 92. VEGF-A may also be an important mediator in the oligovascular niche. VEGF-A is secreted from cerebral endothelial cells and its receptor (Flk-1) is expressed in OPCs 93. In vitro cell culture studies confirmed that endothelial-derived VEGF-A did promote the migration of OPCs 94. Notably, the trophic support from cerebral endothelial cells to OPCs may change under diseased conditions. Even sub-lethal level of oxidative stress could suppress the secretion of FGF-2/BDNF from cerebral endothelial cells, indicating that cerebral endothelial cells would not provide sufficient support to OPCs under diseased conditions 92. Therefore, disruption of OPC-endothelium trophic coupling may hasten the progression of white matter-related diseases.
In turn, cells from the oligodendrocyte lineage may modulate the cerebrovascular system. For example, OPCs produce TGF-β, which maintains the tightness of BBB during development 95. However, under diseased conditions such as cerebral hypoperfusion in the adult brain, OPCs could damage the BBB by secreting matrix metalloproteinase-9 96. Importantly, patterns of cell-cell interaction in the oligovascular niche may change again during the chronic (repairing) phase after injury. After white matter damage, oligodendrocyte-derived MMP-9 would work on white matter remodeling by promoting angiogenesis 97. Taken together, these findings indicate that the oligovascular niche contributes to cellular plasticity in cerebral white matter.
4. Cell-cell interaction in disease
Cell-cell interaction in the perivascular region is an important mechanism for cellular plasticity, which maintains neurovascular homeostasis. Importantly, these cell-cell interactions may change under disease conditions – for example, disruption of cell-cell trophic coupling causes cellular damage, but at the same time, changes in neurovascular interactions after brain injury may promote neurovascular repair. Therefore, an understanding of cell-cell interaction in disease may lead to new therapeutic approaches for CNS diseases. In this section, we will briefly introduce some key findings related to changes in neurovascular components under pathological conditions.
In the acute phase after the cerebral ischemia, injured neurons release damage-associated molecular pattern proteins (DAMPs), leading to the secretion of deleterious mediators such as inflammatory cytokines (e.g. IL-1β and TNF-α) and reactive oxygen species. These factors induce BBB breakdown and facilitate the infiltration of circulating monocytes, neutrophils and lymphocytes 98,99, which then accelerate post-ischemic inflammation, which may further damage the surviving neurons 100. Under ischemic conditions, CX3CL1/CX3CR1 signaling promotes microglial phagocytosis of apoptotic neurons, an important aspect of remodeling101. This response also promotes neurogenesis in the ipsilateral SVZ - residual NSPCs are activated to migrate to the ischemic lesion and to proliferate at that site 102. This dual effect is also observed in other signaling proteins. For example, HMGB-1, which belongs to DAMP family, induces inflammatory/deleterious effects in the early phase of stroke, while it contributes to neurovascular remodeling by promoting neurogenesis and angiogenesis in the chronic recovery phase 103.
Other mechanisms of cell-cell interactions are also affected under conditions of stress. Deficits in axonal energy caused by metabolic disturbance in glial cells are closely related to neurodegenerative disease. Oligodendrocyte dysfunction may cause neuronal/axonal damage. Under normal conditions, oligodendrocytes supply lactate to neurons through monocarboxylate transporter 1 (MCT1) 104. However, in the setting of amyotrophic lateral sclerosis, oligodendrocytes exhibit MCT1 deficiency – a phenomenon observed both in a mouse model and in human subjects 105. This deficit would likely lead to a disruption of the trophic support from oligodendrocytes to neurons (axons).
Protein aggregation and degeneration in glial cells are also related to the progression of neuronal disease. Astrocytes take up and degrade Aβ protein, which is deposited in brains of patients with Alzheimer’s disease (AD) and thought to cause neuronal damage 106. Enhancing the autophagy-mediated proteolysis in astrocytes attenuate Aβ deposit 107, suggesting that astrocytes are important in Aβ clearance and that astrocyte dysfunction may accelerate the progression of AD pathology. In addition to Aβ protein, α-synuclein is another important protein in neurodegenerative diseases. α-synuclein deposits mostly in neuronal Lewy bodies, and α-synuclein-aggregated Lewy neurites are observed in brains of Parkinson’s disease or dementia patients. On the other hand, in multiple system atrophy patients, α-synuclein deposits largely in oligodendroglial cytoplasmic inclusions 108. These findings suggest that mechanisms of α-synuclein clearance could be deranged in specific cell populations depending on the type of disease.
In general, pathological conditions disturb cell-cell trophic coupling, leading to neurovascular dysfunction. But, as briefly mentioned above, cell-cell interactions may recover to some extent during the chronic phase to promote compensative neurogenesis/angiogenesis/oligodendrogenesis. However, it still remains unknown how those cell-cell interactions contribute to barriergenesis (i.e. re-sealing BBB) after brain injury. During development, cells in the perivascular region, such as pericytes and astrocytes, provide support to cerebral endothelial cells in BBB formation 109. Therefore, to reveal the mechanisms of cellular plasticity in the neurovascular unit, future studies are warranted to investigate the roles of pericyte-endothelium or astrocyte-endothelium trophic coupling in compensative barriergenesis of BBB in adult brains.
5. Conclusion
The concept of the neurovascular unit has provided a basic framework for understanding brain physiology and pathology. As discussed in this chapter, cell-cell trophic coupling is important for the regulation of neurovascular function and preservation of brain homeostasis. In this regard, perivascular region may play an essential role because the neurovascular/oligovascular niche mediates the interaction of cerebral endothelial cells with other cells within the neurovascular unit to regulate cellular plasticity. Ultimately, cellular plasticity can be easily affected by neighboring microenvironments in both beneficial and detrimental ways, and therefore, a precise understanding of cell-cell interaction pathways is needed to develop efficient therapeutic treatments for CNS diseases.
References
- 1.del Zoppo GJ. Inflammation and the neurovascular unit in the setting of focal cerebral ischemia. Neuroscience. 2009;158:972–982. doi: 10.1016/j.neuroscience.2008.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacological reviews. 2005;57:173–185. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- 3.Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nature reviews. Neuroscience. 2004;5:347–360. doi: 10.1038/nrn1387. [DOI] [PubMed] [Google Scholar]
- 4.Lo EH, Dalkara T, Moskowitz MA. Mechanisms, challenges and opportunities in stroke. Nat Rev Neurosci. 2003;4:399–415. doi: 10.1038/nrn1106. nrn1106 [pii] [DOI] [PubMed] [Google Scholar]
- 5.Lo EH, Broderick JP, Moskowitz MA. tPA and proteolysis in the neurovascular unit. Stroke; a journal of cerebral circulation. 2004;35:354–356. doi: 10.1161/01.str.0000115164.80010.8a. [DOI] [PubMed] [Google Scholar]
- 6.Zacchigna S, Lambrechts D, Carmeliet P. Neurovascular signalling defects in neurodegeneration. Nature reviews. Neuroscience. 2008;9:169–181. doi: 10.1038/nrn2336. [DOI] [PubMed] [Google Scholar]
- 7.Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. doi: 10.1016/j.neuron.2008.01.003. S0896-6273(08)00034-2 [pii] [DOI] [PubMed] [Google Scholar]
- 8.Ohab JJ, Fleming S, Blesch A, Carmichael ST. A neurovascular niche for neurogenesis after stroke. J Neurosci. 2006;26:13007–13016. doi: 10.1523/JNEUROSCI.4323-06.2006. 26/50/13007 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kokovay E, et al. Adult SVZ lineage cells home to and leave the vascular niche via differential responses to SDF1/CXCR4 signaling. Cell Stem Cell. 2010;7:163–173. doi: 10.1016/j.stem.2010.05.019. S1934-5909(10)00279-1 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taguchi A, et al. Administration of CD34+ cells after stroke enhances neurogenesis via angiogenesis in a mouse model. J Clin Invest. 2004;114:330–338. doi: 10.1172/JCI20622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thored P, et al. Long-term neuroblast migration along blood vessels in an area with transient angiogenesis and increased vascularization after stroke. Stroke. 2007;38:3032–3039. doi: 10.1161/STROKEAHA.107.488445. STROKEAHA.107.488445 [pii] [DOI] [PubMed] [Google Scholar]
- 12.Urich E, Lazic SE, Molnos J, Wells I, Freskgard PO. Transcriptional profiling of human brain endothelial cells reveals key properties crucial for predictive in vitro blood-brain barrier models. PLoS One. 2012;7:e38149. doi: 10.1371/journal.pone.0038149. PONE-D-12-03987 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olah M, et al. Identification of a microglia phenotype supportive of remyelination. Glia. 2012;60:306–321. doi: 10.1002/glia.21266. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y, Barres BA. Astrocyte heterogeneity: an underappreciated topic in neurobiology. Curr Opin Neurobiol. 2010;20:588–594. doi: 10.1016/j.conb.2010.06.005. S0959-4388(10)00102-9 [pii] [DOI] [PubMed] [Google Scholar]
- 15.Zamanian JL, et al. Genomic analysis of reactive astrogliosis. J Neurosci. 2012;32:6391–6410. doi: 10.1523/JNEUROSCI.6221-11.2012. 32/18/6391 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barres BA. The mystery and magic of glia: a perspective on their roles in health and disease. Neuron. 2008;60:430–440. doi: 10.1016/j.neuron.2008.10.013. S0896-6273(08)00886-6 [pii] [DOI] [PubMed] [Google Scholar]
- 17.Allen NJ, Barres BA. Neuroscience: Glia - more than just brain glue. Nature. 2009;457:675–677. doi: 10.1038/457675a. 457675a [pii] [DOI] [PubMed] [Google Scholar]
- 18.Hirabayashi Y, Gotoh Y. Epigenetic control of neural precursor cell fate during development. Nat Rev Neurosci. 2010;11:377–388. doi: 10.1038/nrn2810. [DOI] [PubMed] [Google Scholar]
- 19.Ables JL, Breunig JJ, Eisch AJ, Rakic P. Not(ch) just development: Notch signalling in the adult brain. Nat Rev Neurosci. 2011;12:269–283. doi: 10.1038/nrn3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qian X, et al. Timing of CNS cell generation: a programmed sequence of neuron and glial cell production from isolated murine cortical stem cells. Neuron. 2000;28:69–80. doi: 10.1016/s0896-6273(00)00086-6. [DOI] [PubMed] [Google Scholar]
- 21.Wang MM. Notch signaling and Notch signaling modifiers. Int J Biochem Cell Biol. 2011;43:1550–1562. doi: 10.1016/j.biocel.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alberi L, et al. Activity-induced Notch signaling in neurons requires Arc/Arg3.1 and is essential for synaptic plasticity in hippocampal networks. Neuron. 2011;69:437–444. doi: 10.1016/j.neuron.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zechner D, et al. beta-Catenin signals regulate cell growth and the balance between progenitor cell expansion and differentiation in the nervous system. Dev Biol. 2003;258:406–418. doi: 10.1016/s0012-1606(03)00123-4. [DOI] [PubMed] [Google Scholar]
- 24.Hirabayashi Y, et al. The Wnt/beta-catenin pathway directs neuronal differentiation of cortical neural precursor cells. Development. 2004;131:2791–2801. doi: 10.1242/dev.01165. [DOI] [PubMed] [Google Scholar]
- 25.Israsena N, Hu M, Fu W, Kan L, Kessler JA. The presence of FGF2 signaling determines whether beta-catenin exerts effects on proliferation or neuronal differentiation of neural stem cells. Dev Biol. 2004;268:220–231. doi: 10.1016/j.ydbio.2003.12.024. [DOI] [PubMed] [Google Scholar]
- 26.Sun Y, et al. Neurogenin promotes neurogenesis and inhibits glial differentiation by independent mechanisms. Cell. 2001;104:365–376. doi: 10.1016/s0092-8674(01)00224-0. [DOI] [PubMed] [Google Scholar]
- 27.Nieto M, Schuurmans C, Britz O, Guillemot F. Neural bHLH genes control the neuronal versus glial fate decision in cortical progenitors. Neuron. 2001;29:401–413. doi: 10.1016/s0896-6273(01)00214-8. [DOI] [PubMed] [Google Scholar]
- 28.Hirabayashi Y, et al. Polycomb limits the neurogenic competence of neural precursor cells to promote astrogenic fate transition. Neuron. 2009;63:600–613. doi: 10.1016/j.neuron.2009.08.021. [DOI] [PubMed] [Google Scholar]
- 29.Pinto L, Gotz M. Radial glial cell heterogeneity--the source of diverse progeny in the CNS. Prog Neurobiol. 2007;83:2–23. doi: 10.1016/j.pneurobio.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 30.Namihira M, et al. Committed neuronal precursors confer astrocytic potential on residual neural precursor cells. Dev Cell. 2009;16:245–255. doi: 10.1016/j.devcel.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 31.Kondo T, Raff M. Oligodendrocyte precursor cells reprogrammed to become multipotential CNS stem cells. Science. 2000;289:1754–1757. doi: 10.1126/science.289.5485.1754. [DOI] [PubMed] [Google Scholar]
- 32.Nishiyama A, Komitova M, Suzuki R, Zhu X. Polydendrocytes (NG2 cells): multifunctional cells with lineage plasticity. Nat Rev Neurosci. 2009;10:9–22. doi: 10.1038/nrn2495. nrn2495 [pii] [DOI] [PubMed] [Google Scholar]
- 33.Raff MC, Miller RH, Noble M. A glial progenitor cell that develops in vitro into an astrocyte or an oligodendrocyte depending on culture medium. Nature. 1983;303:390–396. doi: 10.1038/303390a0. [DOI] [PubMed] [Google Scholar]
- 34.Dawson MR, Polito A, Levine JM, Reynolds R. NG2-expressing glial progenitor cells: an abundant and widespread population of cycling cells in the adult rat CNS. Mol Cell Neurosci. 2003;24:476–488. doi: 10.1016/s1044-7431(03)00210-0. [DOI] [PubMed] [Google Scholar]
- 35.Peters A. A fourth type of neuroglial cell in the adult central nervous system. J Neurocytol. 2004;33:345–357. doi: 10.1023/B:NEUR.0000044195.64009.27. [DOI] [PubMed] [Google Scholar]
- 36.Zhu X, Bergles DE, Nishiyama A. NG2 cells generate both oligodendrocytes and gray matter astrocytes. Development. 2008;135:145–157. doi: 10.1242/dev.004895. [DOI] [PubMed] [Google Scholar]
- 37.Franklin RJ, Bayley SA, Milner R, Ffrench-Constant C, Blakemore WF. Differentiation of the O-2A progenitor cell line CG-4 into oligodendrocytes and astrocytes following transplantation into glia-deficient areas of CNS white matter. Glia. 1995;13:39–44. doi: 10.1002/glia.440130105. [DOI] [PubMed] [Google Scholar]
- 38.Windrem MS, et al. Fetal and adult human oligodendrocyte progenitor cell isolates myelinate the congenitally dysmyelinated brain. Nat Med. 2004;10:93–97. doi: 10.1038/nm974. [DOI] [PubMed] [Google Scholar]
- 39.Hill RA, Patel KD, Goncalves CM, Grutzendler J, Nishiyama A. Modulation of oligodendrocyte generation during a critical temporal window after NG2 cell division. Nat Neurosci. 2014;17:1518–1527. doi: 10.1038/nn.3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhu X, et al. Olig2-dependent developmental fate switch of NG2 cells. Development. 2012;139:2299–2307. doi: 10.1242/dev.078873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou Q, Anderson DJ. The bHLH transcription factors OLIG2 and OLIG1 couple neuronal and glial subtype specification. Cell. 2002;109:61–73. doi: 10.1016/s0092-8674(02)00677-3. [DOI] [PubMed] [Google Scholar]
- 42.Lu QR, et al. Common developmental requirement for Olig function indicates a motor neuron/oligodendrocyte connection. Cell. 2002;109:75–86. doi: 10.1016/s0092-8674(02)00678-5. [DOI] [PubMed] [Google Scholar]
- 43.Cai J, et al. A crucial role for Olig2 in white matter astrocyte development. Development. 2007;134:1887–1899. doi: 10.1242/dev.02847. [DOI] [PubMed] [Google Scholar]
- 44.Moyon S, Liang J, Casaccia P. Epigenetics in NG2 glia cells. Brain Res. 2015 doi: 10.1016/j.brainres.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cunliffe VT, Casaccia-Bonnefil P. Histone deacetylase 1 is essential for oligodendrocyte specification in the zebrafish CNS. Mech Dev. 2006;123:24–30. doi: 10.1016/j.mod.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 46.Ye F, et al. HDAC1 and HDAC2 regulate oligodendrocyte differentiation by disrupting the beta-catenin-TCF interaction. Nat Neurosci. 2009;12:829–838. doi: 10.1038/nn.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shen S, et al. Age-dependent epigenetic control of differentiation inhibitors is critical for remyelination efficiency. Nat Neurosci. 2008;11:1024–1034. doi: 10.1038/nn.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.He Y, et al. The transcription factor Yin Yang 1 is essential for oligodendrocyte progenitor differentiation. Neuron. 2007;55:217–230. doi: 10.1016/j.neuron.2007.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ishibashi T, et al. Astrocytes promote myelination in response to electrical impulses. Neuron. 2006;49:823–832. doi: 10.1016/j.neuron.2006.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dugas JC, et al. Dicer1 and miR-219 Are required for normal oligodendrocyte differentiation and myelination. Neuron. 2010;65:597–611. doi: 10.1016/j.neuron.2010.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang S, et al. Notch receptor activation inhibits oligodendrocyte differentiation. Neuron. 1998;21:63–75. doi: 10.1016/s0896-6273(00)80515-2. [DOI] [PubMed] [Google Scholar]
- 52.Hirasawa T, et al. Visualization of microglia in living tissues using Iba1-EGFP transgenic mice. J Neurosci Res. 2005;81:357–362. doi: 10.1002/jnr.20480. [DOI] [PubMed] [Google Scholar]
- 53.Chan WY, Kohsaka S, Rezaie P. The origin and cell lineage of microglia: new concepts. Brain Res Rev. 2007;53:344–354. doi: 10.1016/j.brainresrev.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 54.Harrison JK, et al. Role for neuronally derived fractalkine in mediating interactions between neurons and CX3CR1-expressing microglia. Proc Natl Acad Sci USA. 1998;95:10896–10901. doi: 10.1073/pnas.95.18.10896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim KH, Son SM, Mook-Jung I. Contributions of microglia to structural synaptic plasticity. J Exp Neurosci. 2013;7:85–91. doi: 10.4137/JEN.S11269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schafer DP, et al. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 2012;74:691–705. doi: 10.1016/j.neuron.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pocock JM, Kettenmann H. Neurotransmitter receptors on microglia. Trends Neurosci. 2007;30:527–535. doi: 10.1016/j.tins.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 58.Parkhurst CN, et al. Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell. 2013;155:1596–1609. doi: 10.1016/j.cell.2013.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Paolicelli RC, et al. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333:1456–1458. doi: 10.1126/science.1202529. [DOI] [PubMed] [Google Scholar]
- 60.Elmore MR, et al. Colony-stimulating factor 1 receptor signaling is necessary for microglia viability, unmasking a microglia progenitor cell in the adult brain. Neuron. 2014;82:380–397. doi: 10.1016/j.neuron.2014.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Augustin HG. Tubes, branches, and pillars: the many ways of forming a new vasculature. Circ Res. 2001;89:645–647. [PubMed] [Google Scholar]
- 62.Asahara T, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 63.Goldman SA, Chen Z. Perivascular instruction of cell genesis and fate in the adult brain. Nat Neurosci. 2011;14:1382–1389. doi: 10.1038/nn.2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen J, et al. Endothelial nitric oxide synthase regulates brain-derived neurotrophic factor expression and neurogenesis after stroke in mice. J Neurosci. 2005;25:2366–2375. doi: 10.1523/JNEUROSCI.5071-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Packer MA, et al. Nitric oxide negatively regulates mammalian adult neurogenesis. Proc Natl Acad Sci USA. 2003;100:9566–9571. doi: 10.1073/pnas.1633579100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Licht T, Keshet E. Delineating multiple functions of VEGF-A in the adult brain. Cell Mol Life Sci. 2013;70:1727–1737. doi: 10.1007/s00018-013-1280-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Leventhal C, Rafii S, Rafii D, Shahar A, Goldman SA. Endothelial trophic support of neuronal production and recruitment from the adult mammalian subependyma. Mol Cell Neurosci. 1999;13:450–464. doi: 10.1006/mcne.1999.0762. [DOI] [PubMed] [Google Scholar]
- 68.Torroglosa A, et al. Nitric oxide decreases subventricular zone stem cell proliferation by inhibition of epidermal growth factor receptor and phosphoinositide-3-kinase/Akt pathway. Stem Cells. 2007;25:88–97. doi: 10.1634/stemcells.2006-0131. [DOI] [PubMed] [Google Scholar]
- 69.Maharaj AS, et al. VEGF and TGF-beta are required for the maintenance of the choroid plexus and ependyma. J Exp Med. 2008;205:491–501. doi: 10.1084/jem.20072041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cao L, et al. VEGF links hippocampal activity with neurogenesis, learning and memory. Nat Genet. 2004;36:827–835. doi: 10.1038/ng1395. [DOI] [PubMed] [Google Scholar]
- 71.Rasika S, Alvarez-Buylla A, Nottebohm F. BDNF mediates the effects of testosterone on the survival of new neurons in an adult brain. Neuron. 1999;22:53–62. doi: 10.1016/s0896-6273(00)80678-9. [DOI] [PubMed] [Google Scholar]
- 72.Andreu-Agullo C, Morante-Redolat JM, Delgado AC, Farinas I. Vascular niche factor PEDF modulates Notch-dependent stemness in the adult subependymal zone. Nat Neurosci. 2009;12:1514–1523. doi: 10.1038/nn.2437. [DOI] [PubMed] [Google Scholar]
- 73.Mathiisen TM, Lehre KP, Danbolt NC, Ottersen OP. The perivascular astroglial sheath provides a complete covering of the brain microvessels: an electron microscopic 3D reconstruction. Glia. 2010;58:1094–1103. doi: 10.1002/glia.20990. [DOI] [PubMed] [Google Scholar]
- 74.Armulik A, Genove G, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell. 2011;21:193–215. doi: 10.1016/j.devcel.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 75.Sims DE. The pericyte--a review. Tissue Cell. 1986;18:153–174. doi: 10.1016/0040-8166(86)90026-1. [DOI] [PubMed] [Google Scholar]
- 76.Armulik A, et al. Pericytes regulate the blood-brain barrier. Nature. 2010;468:557–561. doi: 10.1038/nature09522. nature09522 [pii] [DOI] [PubMed] [Google Scholar]
- 77.Bell RD, et al. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron. 2010;68:409–427. doi: 10.1016/j.neuron.2010.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bjarnegard M, et al. Endothelium-specific ablation of PDGFB leads to pericyte loss and glomerular, cardiac and placental abnormalities. Development. 2004;131:1847–1857. doi: 10.1242/dev.01080. [DOI] [PubMed] [Google Scholar]
- 79.Falcon BL, et al. Contrasting actions of selective inhibitors of angiopoietin-1 and angiopoietin-2 on the normalization of tumor blood vessels. Am J Pathol. 2009;175:2159–2170. doi: 10.2353/ajpath.2009.090391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gaengel K, Genove G, Armulik A, Betsholtz C. Endothelial-mural cell signaling in vascular development and angiogenesis. Arterioscler Thromb Vasc Biol. 2009;29:630–638. doi: 10.1161/ATVBAHA.107.161521. [DOI] [PubMed] [Google Scholar]
- 81.Maki T, et al. Potential interactions between pericytes and oligodendrocyte precursor cells in perivascular regions of cerebral white matter. Neurosci Lett. 2015;597:164–169. doi: 10.1016/j.neulet.2015.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hungerford JE, Little CD. Developmental biology of the vascular smooth muscle cell: building a multilayered vessel wall. J Vasc Res. 1999;36:2–27. doi: 10.1159/000025622. 25622. [DOI] [PubMed] [Google Scholar]
- 83.Karow M, et al. Reprogramming of pericyte-derived cells of the adult human brain into induced neuronal cells. Cell stem cell. 2012;11:471–476. doi: 10.1016/j.stem.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 84.Obermeier B, Daneman R, Ransohoff RM. Development, maintenance and disruption of the blood-brain barrier. Nat Med. 2013;19:1584–1596. doi: 10.1038/nm.3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zonta M, et al. Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nat Neurosci. 2003;6:43–50. doi: 10.1038/nn980. [DOI] [PubMed] [Google Scholar]
- 86.Miyamoto N, et al. Crosstalk between cerebral endothelium and oligodendrocyte. Cell Mol Life Sci. 2014;71:1055–1066. doi: 10.1007/s00018-013-1488-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Arai K, Lo EH. Oligovascular signaling in white matter stroke. Biol Pharm Bull. 2009;32:1639–1644. doi: 10.1248/bpb.32.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Levine JM, Reynolds R, Fawcett JW. The oligodendrocyte precursor cell in health and disease. Trends Neurosci. 2001;24:39–47. doi: 10.1016/s0166-2236(00)01691-x. S0166-2236(00)01691-X [pii] [DOI] [PubMed] [Google Scholar]
- 89.Nishiyama A, Chang A, Trapp BD. NG2+ glial cells: a novel glial cell population in the adult brain. J Neuropathol Exp Neurol. 1999;58:1113–1124. doi: 10.1097/00005072-199911000-00001. [DOI] [PubMed] [Google Scholar]
- 90.Chang A, Nishiyama A, Peterson J, Prineas J, Trapp BD. NG2-positive oligodendrocyte progenitor cells in adult human brain and multiple sclerosis lesions. J Neurosci. 2000;20:6404–6412. doi: 10.1523/JNEUROSCI.20-17-06404.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tanaka K, Nogawa S, Suzuki S, Dembo T, Kosakai A. Upregulation of oligodendrocyte progenitor cells associated with restoration of mature oligodendrocytes and myelination in peri-infarct area in the rat brain. Brain Res. 2003;989:172–179. doi: 10.1016/s0006-8993(03)03317-1. [DOI] [PubMed] [Google Scholar]
- 92.Arai K, Lo EH. An oligovascular niche: cerebral endothelial cells promote the survival and proliferation of oligodendrocyte precursor cells. J Neurosci. 2009;29:4351–4355. doi: 10.1523/JNEUROSCI.0035-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hayakawa K, et al. Vascular endothelial growth factor regulates the migration of oligodendrocyte precursor cells. J Neurosci. 2011;31:10666–10670. doi: 10.1523/JNEUROSCI.1944-11.2011. 31/29/10666 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hayakawa K, et al. Cerebral endothelial derived vascular endothelial growth factor promotes the migration but not the proliferation of oligodendrocyte precursor cells in vitro. Neuroscience letters. 2012;513:42–48. doi: 10.1016/j.neulet.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Seo JH, et al. Oligodendrocyte precursor cells support blood-brain barrier integrity via TGF-beta signaling. PLoS One. 2014;9:e103174. doi: 10.1371/journal.pone.0103174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Seo JH, et al. Oligodendrocyte precursors induce early blood-brain barrier opening after white matter injury. J Clin Invest. 2013;123:782–786. doi: 10.1172/JCI65863. 65863 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pham LD, et al. Crosstalk between oligodendrocytes and cerebral endothelium contributes to vascular remodeling after white matter injury. Glia. 2012;60:875–881. doi: 10.1002/glia.22320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jackman K, Iadecola C. Neurovascular regulation in the ischemic brain. Antioxid Redox Signal. 2015;22:149–160. doi: 10.1089/ars.2013.5669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.del Zoppo G, et al. Inflammation and stroke: putative role for cytokines, adhesion molecules and iNOS in brain response to ischemia. Brain Pathol. 2000;10:95–112. doi: 10.1111/j.1750-3639.2000.tb00247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fitch MT, Silver J. CNS injury, glial scars, and inflammation: Inhibitory extracellular matrices and regeneration failure. Exp Neurol. 2008;209:294–301. doi: 10.1016/j.expneurol.2007.05.014. S0014-4886(07)00213-0 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cardona AE, et al. Control of microglial neurotoxicity by the fractalkine receptor. Nat Neurosci. 2006;9:917–924. doi: 10.1038/nn1715. [DOI] [PubMed] [Google Scholar]
- 102.Zhang R, et al. Activated neural stem cells contribute to stroke-induced neurogenesis and neuroblast migration toward the infarct boundary in adult rats. J Cereb Blood Flow Metab. 2004;24:441–448. doi: 10.1097/00004647-200404000-00009. [DOI] [PubMed] [Google Scholar]
- 103.Hayakawa K, Qiu J, Lo EH. Biphasic actions of HMGB1 signaling in inflammation and recovery after stroke. Ann N Y Acad Sci. 2010;1207:50–57. doi: 10.1111/j.1749-6632.2010.05728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Morrison BM, Lee Y, Rothstein JD. Oligodendroglia: metabolic supporters of axons. Trends Cell Biol. 2013;23:644–651. doi: 10.1016/j.tcb.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kang SH, Fukaya M, Yang JK, Rothstein JD, Bergles DE. NG2+ CNS glial progenitors remain committed to the oligodendrocyte lineage in postnatal life and following neurodegeneration. Neuron. 2010;68:668–681. doi: 10.1016/j.neuron.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Guenette SY. Astrocytes: a cellular player in Abeta clearance and degradation. Trends Mol Med. 2003;9:279–280. doi: 10.1016/s1471-4914(03)00112-6. [DOI] [PubMed] [Google Scholar]
- 107.Xiao Q, et al. Enhancing astrocytic lysosome biogenesis facilitates Abeta clearance and attenuates amyloid plaque pathogenesis. J Neurosci. 2014;34:9607–9620. doi: 10.1523/JNEUROSCI.3788-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Halliday GM, Holton JL, Revesz T, Dickson DW. Neuropathology underlying clinical variability in patients with synucleinopathies. Acta Neuropathol. 2011;122:187–204. doi: 10.1007/s00401-011-0852-9. [DOI] [PubMed] [Google Scholar]
- 109.Siegenthaler JA, Sohet F, Daneman R. ‘Sealing off the CNS’: cellular and molecular regulation of blood-brain barriergenesis. Curr Opin Neurobiol. 2013;23:1057–1064. doi: 10.1016/j.conb.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]