Fig. 2. The molecular structure, dynamic surface tension, and impact behavior of SDS, TSs, and AOT.
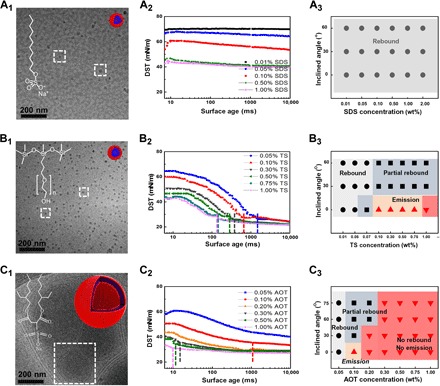
(A1 and B1) Cryo-TEM images of both 1% SDS and 1% TSs show the micelle aggregates at a mass fraction of 1%. (C1) Cryo-TEM image of 1% AOT shows that multilamellar vesicles have formed, indicating the dense aggregates of AOT molecules at the air/water interface. (A2) SDS has a long chain, and its dynamic surface tension (DST) slowly falls in the rapid fall region and cannot reach its equilibrium within a surface age of 100 ms. (A3) The droplets containing SDS are easy to rebound on the superhydrophobic surface even for highly concentrated solution. (B2) TSs have long induction periods in the first tens or hundreds of seconds. (B3) With the increase of the concentration, the induction period is shortened so that the impact behavior turns from bouncing, emission, to no rebound. On the inclined surface, it is still easy to partially rebound. (C2) AOT distinguishes itself from other surfactants in the fastest diffusion speed to form a densely packed molecular layer at the air/water interface. AOT directly approaches low surface tension of around 32 mN/m at the very beginning of the bubble pressure measurement, showing the lowest DST among the three surfactants within a surface age of 80 ms. The DSTs of 1% SDS and 1% TSs begin nearly at the same value of ~43 mN/m. TS quickly decreases to a lower value, and SDS slowly decreases to a higher value. (C3) AOT is the most effective surfactant that inhibits bouncing of the liquid drops on the superhydrophobic surface.
