Summary
The objective of this study was to evaluate the determinants of long-term adherence to positive airway pressure (PAP) treatment among obstructive sleep apnea (OSA) patients with special emphasis on patients who stop PAP treatment within one year.
This is a prospective long-term follow-up of subjects in the Icelandic Sleep Apnea Cohort (ISAC) who were diagnosed with OSA between the years 2005-2009 and started on PAP treatment. In October 2014, PAP adherence was obtained by systematically evaluating available clinical files (n=796; 644 males, 152 females) with moderate to severe OSA (apnea-hypopnea index ≥15 events/hr).
The mean follow-up time was 6.7±1.2 years. In total, 123 subjects (15.5%) returned their PAP device within the first year, 170 (21.4%) returned it later and 503 (63.2%) were still using PAP. The quitters within the first year had lower body mass index (BMI), milder OSA, less sleepiness and more often had symptoms of initial and late insomnia compared to long-term PAP users at baseline. Both initial and late insomnia were after adjustment still significantly associated with being an early quitter among subjects with BMI < 30 kg/m2 but not among those with BMI ≥ 30 kg/m2. The prevalence of early quitters decreased significantly during the study period (2005-2009).
Almost two-thirds of moderate to severe OSA patients are PAP users after 7 years. Obesity level, OSA severity and daytime sleepiness are important determinants of long-term adherence. Symptoms of initial and late insomnia are associated with early quitting on PAP among non-obese subjects.
Keywords: Obstructive sleep apnea, obesity, compliance, sleep disturbances
Introduction
Obstructive sleep apnea (OSA) is a chronic disorder characterized by repeated episodes of partial or complete upper airway collapse during sleep (Weaver and Sawyer 2010; Punjabi 2008; Bouloukaki et al. 20214). This can lead to excessive daytime sleepiness, impaired cognition, mood alterations, decreased functional capacity, and increased risk of cardiovascular disease and motor vehicle crashes (Weaver and Sawyer 2010; Punjabi 2008; Bouloukaki et al. 20214). Positive airway pressure (PAP) therapy has been established as the primary treatment for patients with moderate to severe OSA (Weaver and Sawyer 2010; Bouloukaki et al. 2014; Sawyer et al. 2011). The effectiveness of PAP treatment significantly depends on patient adherence to the therapy and lack of PAP adherence is a well-recognized limitation of the effectiveness of OSA treatment (Weaver and Sawyer 2010; Bouloukaki et al. 2014; Sawyer et al. 2011; Weaver and Grunnstein 2008; Weaver 2006; Wohlgemuth et al. 2015; Jurado-Gamez et al. 2015). Patient adherence during the first month after initiation of PAP therapy is a powerful predictor of long term adherence (DeMolles et al. 2014), and the first week of PAP treatment can be critically influential on the patient decision to use PAP (Weaver et al. 1997). Several studies have pointed out the need to identify patients who are at the greatest risk for quitting PAP treatment in order to be able to develop a more personalized approach to improve overall adherence (Sawyer et al. 2011; Rose 2006; Sawyer et al. 2014).
A limited number of studies address long-term adherence to PAP (Bouloukaki et al. 2014; Salepci et al. 2013; Zeller et al. 2013; Galatke et al. 2011; Schoch et al. 2014; Kohler et al. 2010) but available studies indicate that increased OSA severity is associated with greater PAP adherence (Bouloukaki et al. 2014; Galatke et al. 2011; Schoch et al. 2014).
Obesity is an important risk factor for OSA and often associated with more severe OSA (Yong et al. 1985). However, studies by Galetke et al. (2011) and Kohler et al. (2010) did not find obesity to be associated with long term PAP adherence. Furthermore, in a study by Schoch et.al.(2014) overweight patients (BMI between 27-30.5 kg/m2) were more likely to adhere to PAP than leaner patients and obese patients (BMI over 30.5). Symptoms of insomnia have also been associated with worse PAP adherence. Our previous study (Bjornsdottor et al. 2012) indicated that symptoms of initial and late insomnia do not improve on PAP treatment and these symptoms negatively affect PAP adherence. Others have reported similar results, especially showing patients who have difficulties falling asleep to have worse adherence to PAP treatment (Salepci et al. 2013). There is, however, a lack of studies comparing simultaneously OSA severity, obesity, sleepiness, and other symptoms related to OSA in those who quit PAP early and those who manage to use PAP for long term.
The aims of this study were therefore to review long term PAP adherence in a well-defined cohort of OSA patients and to identify groups that are at risk for stopping PAP treatment with a special emphasis on those who quit treatment within the first year (early quitters).
Materials and methods
All patients diagnosed with moderate to severe OSA (apnea-hypopnea index [AHI] ≥15 events/hour) referred to the Department of Respiratory Medicine and Sleep, Landspitali – The National University Hospital (LSH) of Iceland for treatment with PAP from September 2005 to December 2009 were invited to participate in the Icelandic Sleep Apnea Cohort (ISAC) study (Bjornsdottir et al. 2012; Arnardottir et al. 2013; Bjornsdottir et al. 2013; Arnardottir et al. 2013; Bjornsdottir et al. 2015). No other inclusion or exclusion criteria were used. Over 90% of eligible and approached subjects agreed to participate in the prospective cohort at baseline and started PAP treatment.
All PAP treatment in Iceland is administered by the Department of Respiratory Medicine and Sleep at LSH. All participants in the ISAC cohort study started treatment with an auto-adjusting PAP or continuous PAP (CPAP) device (ResMed Corp. San Diego, California, USA) and were only changed to bi-level PAP (BiPAP) or adaptive servo ventilation if treatment efficacy was inadequate, defined by AHI≥15 while using PAP and/or persistent patient complaints. As part of routine care, all OSA patients paid a monthly usage fee (22 US$ per month) for their PAP device, had access to the outpatient clinic and were in a regularly updated registry. Various mask types and humidifiers were available for all patients on PAP. Since 2009 all PAP devices had heated in-line humidifiers.
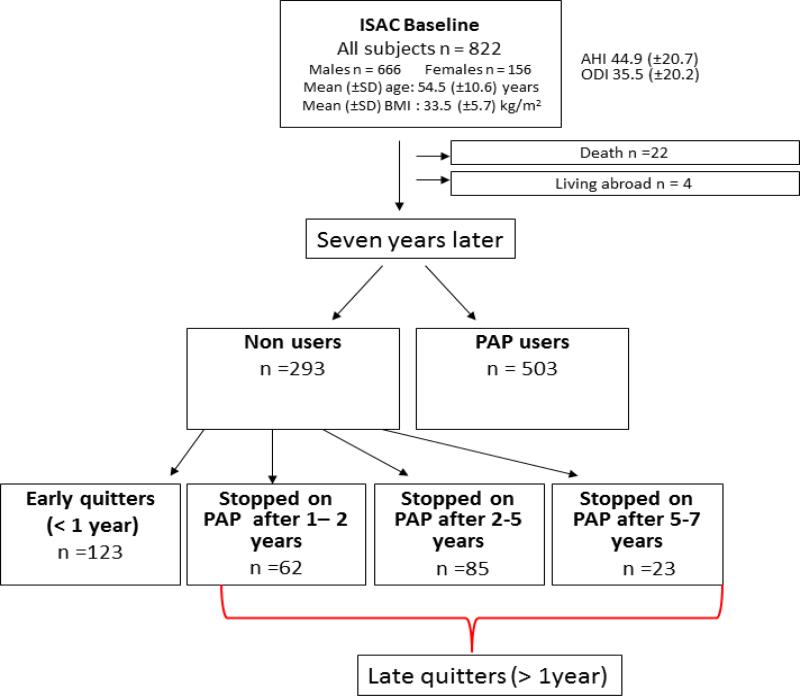
The electronic medical files of all 822 patients in the ISAC study were reviewed in October 2014. A total of 22 patients had died and four patients were living abroad. The files of the 796 remaining patients were reviewed for their last sleep clinic visit and whether they had returned their PAP device. Long term PAP users were defined as those still having a PAP device and paying the monthly user fee. Patients who had stopped their PAP treatment were divided into four groups (Figure 1): early PAP quitters (stopped using PAP within one year), those who stopped using PAP between 1-2 years (n= 62), after 2-4 years (n= 85), and after 5-7 years (n= 23); all these groups except the early quitters were later combined into late quitters as no differences were found between the groups in baseline characteristics (data not shown). The focus of the current study was to compare early PAP quitters and long term PAP users.
Figure 1.
Flow chart of the study population showing PAP use 5-9 years after treatment initiation.
The ISAC study was approved by the Iceland's National Bioethics Committee, the Data Protection Authority of Iceland and the Institutional Review Board of the University of Pennsylvania.
Measurements and Questionnaires
Before participants started PAP treatment, written informed consent was obtained and patients answered standardized questionnaires about their health and sleep. These questionnaires included questions about whether they had hypertension and/or diabetes (doctor diagnosis and medication), as well as cardiovascular disease (CVD) defined as a medical diagnosis of myocardial infarction or heart attack, heart failure and/or stroke. The Epworth Sleepiness Scale (ESS) was used to evaluate daytime sleepiness; participants with ESS score ≥10 were considered to have excessive daytime sleepiness (Johns 1992).
Sleep symptoms were assessed using the Basic Nordic Sleep Questionnaire, which includes questions on insomnia, nocturnal sweating, daytime sleepiness and sleep bruxism (Partinen and Gislason 1995). The response alternatives for those questions were on a frequency scale of 1–5: (1) never or very seldom, (2) less than once a week, (3) once to twice a week, (4) 3–5 times a week, and (5) every day or almost every day of the week. Those scoring 4 or 5 were defined as having the symptom (Partinen and Gislason 1995). Insomnia symptoms were defined into three subtypes; difficulty initiating sleep (initial insomnia), difficulty maintaining sleep (middle insomnia) and early morning awakenings (late insomnia), for more details see our previous publication (Bjornsdottir et al. 2013). The Short Form Health Survey (SF-12) was used to assess quality of life. Both physical and mental health summary scores were derived from the SF-12. These scores range from 0-100 where a score of zero indicates the lowest life quality and 100 indicates the highest life quality (Ware et al. 1996). Questions on symptoms of restless legs syndrome (RLS) were based on recommendations from the International Restless Legs Syndrome Study Groups (Allen et al. 2003).
Sleep recording in ISAC cohort
Prior to referral for PAP treatment, all OSA patients had a type 3 sleep study performed with eitheran Embletta portable monitor, an Embla 12 channel system (Natus Medical Inc., Ontario, Canada), or a Nox T3 device (Nox Medical, Reykjavik, Iceland). The same signals were recorded on all studies: nasal airflow by cannula, oxygen saturation, heart rate, respiratory movements by respiratory inductance plethysmography (RIP) belts, body position and activity. Trained sleep technologists scored all sleep studies and the studies had to have ≥4 hours of a scorable O2 saturation signal. The AHI was calculated as the mean number of apneas and hypopneas per hour of recording (excluding upright time). The oxygen desaturation index (ODI) was calculated as the number of transient drops in oxygen saturation ≥4% per hour of recording. For further details, see previous publications (Bjornsdottir et al. 2013; Arnardottir et al. 2013; Bjornsdottir et al. 2015).
Statistical analyses
All statistical analyses were performed in STATA, version 13.0 (Stata Corporation, College Station, TX). For bivariate analysis, the chi-square test and t-test were used for nominal and continuous variables respectively. Logistic regression was used in adjusted analyses and results are presented as adjusted β-estimates and 95% confidence intervals or adjusted least squares mean estimates and standard errors. The data was square root transformed for statistical analysis as appropriate to normalize distributions.
Results
Among the 796 subjects in this study (males = 644, females = 152), the mean follow-up time was 6.7 ± 1.2 years. Altogether, 36.8% (n = 293) had stopped using the PAP device and 63.2% (n=503) were still on PAP treatment. Non-adherent patients were divided into two groups; early quitters who stopped using PAP < 1 year (n = 123), and late quitters who stopped on PAP after ≥ 1 year (n = 170) (Figure 1).
Different characteristics of early quitters and long term PAP users
The characteristics of the two groups differed at baseline. Those who stopped using their PAP device in the first year after initiation had a lower body mass index (BMI), milder OSA, less daytime sleepiness and reported less often nocturnal sweating, as well as a lower prevalence of hypertension, compared to long term PAP users (Table 1). No differences were found in age, quality of life, prevalence of cardiovascular disease or diabetes, or symptoms of nasal airway restriction between the groups.
Table 1.
Baseline characteristics of the study group (n=796). Data is shown as mean ± standard deviation unless otherwise specified.
| Early quitters (< 1 year) n=123 |
Late quitters (1-7 years) n=170 |
Long term PAP users n=503 |
P value* | |
|---|---|---|---|---|
| Male % | 76.4 | 79.4 | 82.5 | 0.04 |
| Age (years) | 55.0 ± 9.4 | 53.0 ± 10.2 | 54.4 ± 10.8 | 0.74 |
| BMI (kg/m2) | 31.3 ± 5.3 | 33.3 ± 6.3 | 34.1 ± 5.5 | <0.001 |
| AHI (events/h) | 34.2 ± 18.3 | 42.1 ± 18.2 | 48.3 ± 21.1 | <0.001 |
| ODI (events/h) | 26.4 ± 17.0 | 31.1 ± 18.0 | 39.0 ± 20.7 | <0.001 |
| Hypoxia time (minutes SaO2 <90%) | 30.5 ± 53.0 | 39.5 ± 55.1 | 64.9 ± 79.1 | 0.001 |
| Minimum SaO2 (%) | 79.2 ± 7.8 | 77.2 ± 7.3 | 75.3 ± 8.1 | <0.001 |
| ESS total score | 10.1 ± 4.6 | 11.9 ± 5.1 | 12.0 ± 5.1 | <0.001 |
| Mental quality of life (SF-12) | 48.2 ± 11.0 | 48.3 ± 11.3 | 48.5 ± 10.8 | 0.97 |
| Physical quality of life (SF-12) | 41.6 ± 10.3 | 40.4 ± 11.4 | 40.2 ± 10.7 | 0.20 |
| Hypertension (%) | 36.1 | 41.8 | 48.5 | 0.009 |
| Diabetes (%) | 5.8 | 9.4 | 8.4 | 0.20 |
| Cardiovascular disease (%) | 17.2 | 18.8 | 18.0 | 0.46 |
| Nocturnal sweating (%) | 23.6 | 32.9 | 33.0 | 0.03 |
| Blocked nose ≥3 times/week (%) | 35.0 | 34.7 | 35.9 | 0.97 |
BMI= body mass index, AHI= apnea-hypopnea index, ODI= oxygen desaturation index, ESS= Epworth sleepiness scale, SaO2 = Oxygen saturation, PAP = positive airway pressure, SF-12 = Short Form 12. Significance is marked as bold.
P values are shown for difference between early quitter and long term PAP users. The data was square root transformed for statistical analysis as appropriate to normalize distribution
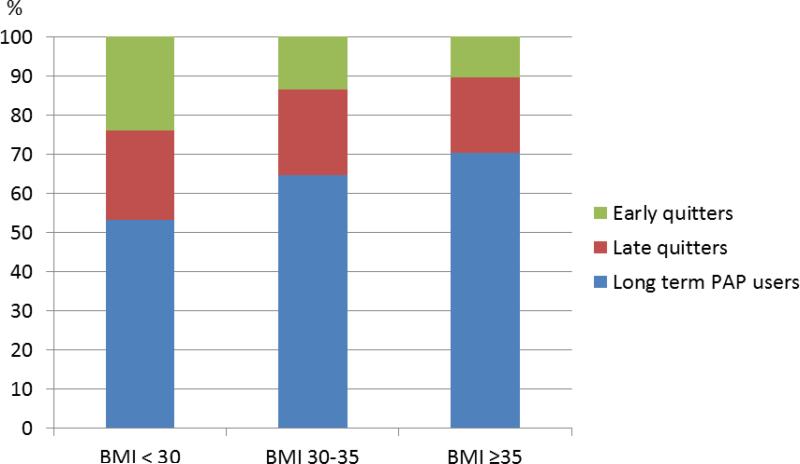
Subjects with a higher BMI were more likely to stay on PAP treatment and being an early quitter was most prevalent among subjects with BMI < 30. The prevalence of being a long term PAP user was 53.1% among subjects with BMI < 30 vs. 70.3% among those with BMI ≥ 35 (p<0.001). Furthermore, 23.9% of subjects with BMI < 30 stopped PAP treatment during the first year vs. 10.3% among subjects with BMI ≥ 35 (Figure 2). A higher ESS score was related to increased PAP adherence, independent of OSA severity.
Figure 2.
The prevalence (%) of early quitters, late quitters and long term PAP users in different body mass index (BMI) categories.
When looking at baseline sleep related symptoms, early quitters were more likely to suffer from initial and late insomnia as well as sleep bruxism compared to long-term PAP users (Table 2). However, no differences were found in the prevalence of RLS. There was not a significant difference in any of the sleep related symptoms between late quitters and long term PAP users.
Table 2.
Modifiable symptoms among early quitters compared to long-term PAP users.
| PAP groups | ||||
|---|---|---|---|---|
| Early quitters (< 1 year) n=123 |
Late quitters (1-7 years) n=170 |
Long term PAP users n=503 |
P value* | |
| Initial insomnia (%) | 23.6 | 14.7 | 13.4 | 0.002 |
| Middle insomnia (%) | 55.3 | 54.1 | 59.6 | 0.38 |
| Late insomnia (%) | 35.8 | 28.4 | 25.5 | 0.01 |
| Sleep bruxism (%) | 25.6 | 19.7 | 16.1 | 0.03 |
| Restless leg syndrome (%) | 39.0 | 34.7 | 37.1 | 0.74 |
PAP = positive airway pressure. Significance is marked as bold.
P values are shown for difference between early quitter and long term PAP users.
Since the above results show that the most distinctive difference between these three groups (early quitters, late quitters, and long term PAP users) is between early quitters and long term PAP users, late quitters were dropped from all following analyses.
Association of insomnia, restless legs and sleep bruxism with being an early PAP quitter for different BMI categories
Logistic regression was performed to explore the adjusted associations of modifiable symptoms with being an early quitter for subjects in different BMI categories. The covariates that was adjusted for in the model were selected a priori, these were AHI score, ESS score, age and gender. Initial insomnia and late insomnia were significantly associated with being an early quitter among subjects with BMI < 30. In more obese subjects none of these symptoms were associated with early quitting after adjustment. Among the most obese subjects (BMI> 35), those with restless leg syndrome or either middle or late insomnia were less likely to be early quitters (Table 3 and Table S1 in supplement).
Table 3.
Adjusted associations of insomnia, restless leg syndrome and sleep bruxism with being an early PAP quitter for different BMI categories.
| Adj. OR (95% CI) * | ||||
|---|---|---|---|---|
| All subjects | BMI< 30 N=183 |
BMI 30-35 N=216 |
BMI > 35 N=227 |
|
| Initial insomnia | 2.03 (1.17-3.52) | 2.21 (1.26-3.86) | 0.58 (0.34-0.99) | 0.43 (0.24-0.77) |
| Middle insomnia | 0.99 (0.63-1.56) | 1.01 (0.64-1.59) | 0.61 (0.36-1.03) | 0.45 (0.25-0.82) |
| Late insomnia | 1.75 (1.09-2.82) | 1.70 (1.05-2.74) | 0.60 (0.35-1.02) | 0.48 (0.26-0.86) |
| Sleep bruxism | 1.75 (0.91 -3.34) | 1.84 (0.96-3.54) | 0.55 (0.29-1.04) | 0.51 (0.25-1.04) |
| Restless leg syndrome | 1.41 (0.88-2.25) | 1.43 (0.89-2.29) | 0.59 (0.37-1.60) | 0.46 (0.25-0.83) |
Adjusted for OSA severity (AHI), daytime sleepiness (ESS score), age and gender.
OSA = obstructive sleep apnea, AHI= apnea-hypopnea index, ESS= Epworth sleepiness scale. Significance is marked as bold.
Changes in PAP adherence during the study period
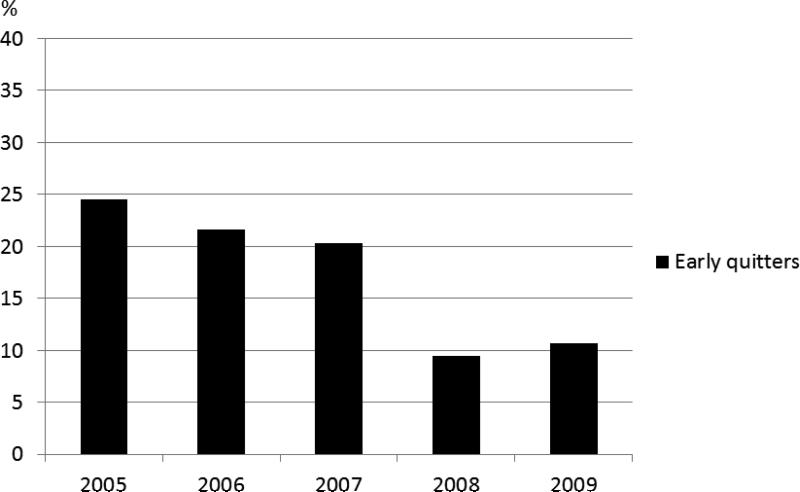
The prevalence of early quitters decreased between years during the study period from 2005 to 2009. Of those who started on PAP in 2005, 24.5% were early quitters compared to 10.7 % of those who started in 2009 (p = 0.001; Figure 3).
Figure 3.
The prevalence (%) of early quitters shown by the year of treatment initiation.
Those who started treatment later in the study period had a higher BMI, were somewhat sleepier, and had more severe OSA compared to subjects who started PAP treatment earlier in the period (Table S2). However, after adjusting for AHI, BMI, ESS score, age, and gender, there was still a significant association between the year that PAP treatment was initiated and PAP adherence (p=0.03).
Discussion
In our well-defined large cohort of adults with OSA, almost two thirds of the subjects were long-term PAP users after an average of 7 years. These long term users had a higher BMI, more severe OSA, more daytime sleepiness, and a higher prevalence of hypertension at baseline than those who quit PAP treatment. Furthermore, subjects who stopped using PAP within one year of treatment initiation were more likely to report baseline symptoms of initial and late insomnia after adjusting for OSA severity and daytime sleepiness. When looking at these associations in different BMI categories, we found that symptoms of initial and late insomnia were associated to non-adherence among non-obese subjects (BMI<30) but not among obese subjects (BMI≥30). This indicates that personalized interventions aimed at improving symptoms of insomnia might potentially improve PAP adherence for non-obese OSA patients.
The strengths of this study are the size of the cohort and the long term follow-up. The data are nationwide as LSH is the sole provider of PAP treatment in Iceland and all OSA patients are in a continuously updated register. The sample is clinical with various comorbidities representing the whole spectrum of OSA patients. Follow-up data were obtained in almost all subjects. A limitation of this study is the lack of precise PAP usage data, we knew which patients had a PAP device and paid the monthly fee, but not the hours per night the patients used the device. Given the magnitude of the fee, it seems reasonable to assume that individuals paying this are continuing to use PAP. This is, moreover a well-defined group of 796 patients that has been followed precisely over time. The majority of these patients came in for a 2 year follow up where their adherence was monitored objectively via PAP download for the last month of usage (Bjornsdottir et al. 2013; Arnardottir et al. 2013). At this two year follow up the majority were PAP users and according to objective data from memory cards the average (± SD) use per night was 6.2 hours ± 2.0 (Bjornsdottir et al. 2013; Arnardottir et al. 2013).
Our results regarding the overall long term adherence to PAP treatment are similar to data reported from McArdle et al. (1999) where the 5 year adherence to PAP was 68% among 1155 patients. Galetke et al. (2011) and Salepci et al. (2013) had similar results but other studies have reported both lower (Kribbs et al. 1993) and higher adherence (Bouloukaki et al. 2014; Zeller et al. 2013; Kohler et al. 2010).
Previous studies have also shown that higher OSA severity is strongly associated with long term PAP adherence (Galetke et al. 2011; Schoch et al. 2014; Kohler et al. 2010). The relationship between long-term PAP adherence and obesity has been unclear; some studies find increased adherence with higher BMI (McArdle et al. 1999; Kribbs et al. 1993; Gagnadoux et al. 2011) after adjustment for OSA severity similar to our findings. In one study the best adherence was among overweight subjects (Schoch et al. 2014), while others have found no relationship with BMI (Galetke et al. 2011; Kohler et al. 2010). In line with results from Schoch et al. (2014) we also found an independent association between long term PAP adherence and increased ESS score at baseline, but this is unlike most other studies (Zeller et al. 2013; Galetke et al. 2011; Kohler et al. 2010) which have not found a relationship with the ESS score independent of OSA severity.
Sleep bruxism was related to early quitting in our unadjusted model, but when adjusting for BMI, OSA severity, daytime sleepiness, age, and gender, sleep bruxism was no longer significant. Symptoms of RLS were not related to PAP non-adherence in our study as has been previously shown (Alepci et al. 2013) but the most obese subjects with RLS in our study were less likely to quit PAP during first year of treatment. Symptoms of initial and late insomnia were related to early quitting among non-obese subjects in our study after adjusting for OSA severity, daytime sleepiness, age and gender. This is in agreement with our previous publications from the ISAC study (Bjornsdottir et al. 2013). Evaluation of insomnia among all OSA patients starting PAP and treating symptoms of initial and late insomnia if present could potentially increase initial PAP adherence among non-obese patients.
In our study there was an improvement in adherence during the study period (2005-2009) as those who started more recently on PAP were less likely to become early quitters. Possible explanations for the increased PAP adherence over the years are technical improvements in PAP equipment with less noisy machines and more comfortable masks, general use of nasal masks and nasal pillows vs. full face masks as well as increased use of heated humidifiers (Zeller et al. 2013; Sommer et al. 2014). There were no changes in patients selection criteria for PAP treatment at the hospital during the study period. The PAP initiation process did not go through any formal changes and no interventions were introduced in an attempt to increase PAP adherence. Increased experience among the nurses providing support for PAP initiation could possibly have influenced the adherence but no data exist regarding that factor.
In summary almost two-thirds of moderate to severe OSA patients are regular PAP users after an average of 7 years. Obesity, OSA severity and sleepiness are all important determinant of long-term adherence but symptoms of initial and late insomnia are related to early quitting on PAP among non-obese subjects.
Supplementary Material
Statement of significance.
This study is important since it evaluates the determinants of long term adherence to PAP treatment and puts a special focus on subjects who stop treatment in the first year. Previous studies have indicated that PAP adherence is often poor and therefore it is important to identify factors that negatively affect adherence and find ways to increase PAP use. Our results show that patients with a higher BMI, more severe OSA and daytime sleepiness are likely to adhere to PAP treatment. Leaner subjects with symptoms of insomnia are on the other hand at risk of discontinuing PAP and may need additional treatment interventions. Future studies should focus on interventions aimed at insomnia symptoms among OSA patients with a lower BMI.
Acknowledgments
Support: NIH grant HL72067 for “A Family Linkage Study of Obstructive Sleep Apnoea” and HL94307 for “Endophenotypes of Sleep Apnea and Role of Obesity”.
Footnotes
This work was performed at Landspítali - The National University Hospital of Iceland.
Conflicts of interest: S.T. Kuna receives grant support from Philips Respironics. E.S. Arnardottir is a consultant for Nox medical, Reykjavík, Iceland. Other authors report no conflicts of interest.
Author contributorship: BE and EB are responsible for the writing of the paper, EB performed the statistical analysis and all authors contributed to the data collection and reviewed the paper at several stages in the writing process.
References
- 1.Allen RP, Picchietti D, Hening WA, Trenkwalder C, Walters AS, Montplaisi J. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med. 2003;4:101–19. doi: 10.1016/s1389-9457(03)00010-8. [DOI] [PubMed] [Google Scholar]
- 2.Arnardottir ES, Maislin G, Jackson N, et al. The role of obesity, different fat compartments and sleep apnea severity in circulating leptin levels: the Icelandic Sleep Apnea Cohort study. Int J Obes (Lond) 2013;37:835–42. doi: 10.1038/ijo.2012.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arnardottir ES, Janson C, Bjornsdotttir E, et al. Nocturnal sweating--a common symptom of obstructive sleep apnoea: the Icelandic sleep apnoea cohort. BMJ Open. 2013;3:e002795. doi: 10.1136/bmjopen-2013-002795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bjornsdottir E, Janson C, Gislason T, et al. Insomnia in untreated sleep apnea patients compared to controls. J Sleep Res. 2012;21:131–8. doi: 10.1111/j.1365-2869.2011.00972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bjornsdottir E, Janson C, Sigurdsson JF, et al. Symptoms of insomnia among OSA patients before and after 2 years of PAP treatment. Sleep. 2013;36:1901–9. doi: 10.5665/sleep.3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Björnsdottir E, Keenan BT, Eysteinsdottir B, et al. Quality of life among untreated sleep apnea patients compared with the general population and changes after treatment with positive airway pressure. J Sleep Res. 2015;24:328–38. doi: 10.1111/jsr.12262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bouloukaki I, Giannadaki K, Mermigkis C, et al. Intensive versus standard follow-up to improve continuous positive airway pressure compliance. Eur Respir J. 2014;44:1262–74. doi: 10.1183/09031936.00021314. [DOI] [PubMed] [Google Scholar]
- 8.DeMolles DA, Sparrow D, Gottlieb DJ, Friedman R. A Pilot Trial of a telecommunications system in sleep apnea management. Med Care. 2014;4288:764–9. doi: 10.1097/01.mlr.0000132353.99209.fe. [DOI] [PubMed] [Google Scholar]
- 9.Gagnadoux F, Le Vaillant M, Goupil F, et al. Influence of marital status and employment status on long-term adherence with continuous positive airway pressure in sleep apnea patients. PLoS One. 2011;6:e22503. doi: 10.1371/journal.pone.0022503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galetke W, Puzzo L, Priegnitz C, Anduleit N, Randerath WJ. Long-term therapy with continuous positive airway pressure in obstructive sleep apnea: adherence side effects and predictors of withdrawal- A real-life study. Respiration. 2011;82:155–61. doi: 10.1159/000322838. [DOI] [PubMed] [Google Scholar]
- 11.Johns MW. Reliability and factor analysis of the Epworth Sleepiness Scale. Sleep. 1992;15:376–81. doi: 10.1093/sleep/15.4.376. [DOI] [PubMed] [Google Scholar]
- 12.Jurado-Gamez B, Bardwell WA, Cordova-Pacheco LJ, Garcia-Amores M, Feu-Collado N, Buela-Casal G. A basic intervention improves CPAP adherence in sleep apnoea patients: a controlled trial. Sleep Breath. 2015;19:509–14. doi: 10.1007/s11325-014-1038-1. [DOI] [PubMed] [Google Scholar]
- 13.Kohler M, Smith D, Tippett V, Strading JR. Predictors of long-term compliance with continuous positive airway pressure. Thorax. 2010;65:829–32. doi: 10.1136/thx.2010.135848. [DOI] [PubMed] [Google Scholar]
- 14.Kribbs NB, Pack AI, Kline LR, et al. Objective measurement of patterns of nasal CPAP use by patients with obstructive sleep apnea. Am Rev Respir Dis. 1993;147:887–95. doi: 10.1164/ajrccm/147.4.887. [DOI] [PubMed] [Google Scholar]
- 15.McArdle N, Devereux G, Heidamejad H, Engleman HM, Mackay TW, Douglas NJ. Long-term use of CPAP therapy for sleep apnea/hypopnea syndrome. Am J Respir Crit Care Med. 1999;159:1108–14. doi: 10.1164/ajrccm.159.4.9807111. [DOI] [PubMed] [Google Scholar]
- 16.Partinen M, Gislason T. Basic Nordic Sleep Questionnaire (BNSQ): a quantitated measure of subjective sleep complaints. J Sleep Res. 1995;4:150–155. doi: 10.1111/j.1365-2869.1995.tb00205.x. [DOI] [PubMed] [Google Scholar]
- 17.Punjabi NM. The epidemiology of adult obstructive sleep apnea. Pro Am Thorac Soc. 2008;5:136–43. doi: 10.1513/pats.200709-155MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rose MV. Positive airway pressure adherence: problems and interventions. Sleep Med Clin. 2006;1:533–39. [Google Scholar]
- 19.Salepci B, Caglayan B, Kiral N, et al. CPAP adherence of Patients with Obstructive Sleep Apnea. Respir Care. 2013;58:1467–73. doi: 10.4187/respcare.02139. [DOI] [PubMed] [Google Scholar]
- 20.Sawyer AN, King TS, Hanlon A, et al. Risk assessment for CPAP nonadherence in adults with newly diagnosed obstructive sleep apnea: preliminary testing of the Index for Nonadherence to PAP (I-NAP). Sleep Breath. 2011;18:875–83. doi: 10.1007/s11325-014-0959-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sawyer AM, King TS, Sawyer DA, Rizzo A. Is inconsistent pre-treatment bedtime related to CPAP non-adherence? Res Nurs Health. 2014;37:504–11. doi: 10.1002/nur.21631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schoch OD, Baty F, Niedermann J, Rudiger JJ, Brutsche MH. Baseline predictors of adherence to positive airway pressure therapy for sleep apnea: A 10-year single-center observational cohort study. Respiration. 2014;87:121–8. doi: 10.1159/000354186. [DOI] [PubMed] [Google Scholar]
- 23.Sommer JU, Kraus M, Birk R, Schultz JD, Hörmann K, Stuck BA. Functional short- and long-term effects of nasal CPAP with and without humidification on the ciliary function of the nasal respiratory epithelium. Sleep Breath. 2014;18:85–93. doi: 10.1007/s11325-013-0853-0. [DOI] [PubMed] [Google Scholar]
- 24.Ware JE, Kosinski M, Keller SD. A 12-item Short-Form Health Survey-construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–33. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 25.Weaver TE, Kribbs NB, Pack AI, et al. Night-to night variability in CPAP use over the first three months of treatment. Sleep. 1997;20:278–83. doi: 10.1093/sleep/20.4.278. [DOI] [PubMed] [Google Scholar]
- 26.Weaver TE. Adherence to positive airway pressure therapy. Curr Opin Pulm Med. 2006;12:409–13. doi: 10.1097/01.mcp.0000245715.97256.32. [DOI] [PubMed] [Google Scholar]
- 27.Weaver TE, Grunnstein R. Adherence to continuous positive airway pressure therapy. Am Thorac Soc. 2008;5:173–78. doi: 10.1513/pats.200708-119MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weaver TE, Sawyer AM. Adherence to continuous positive airway pressure treatment for obstructive sleep apnea: Implications for future interventions. Indian J. Med Res. 2010;131:245–58. [PMC free article] [PubMed] [Google Scholar]
- 29.Wohlgemuth WK, Chirinos DA, Domingo S, Wallace DM. Attempters, adherences, and non-adherers: Latent profile analysis of CPAP use with correlates. Sleep Med. 2015;16:336–42. doi: 10.1016/j.sleep.2014.08.013. [DOI] [PubMed] [Google Scholar]
- 30.Young T, Peppard PE, Taheri S. Excess weight and sleep-disordered breathing. J. Appl Physiol. 2005;99:1592–9. doi: 10.1152/japplphysiol.00587.2005. [DOI] [PubMed] [Google Scholar]
- 31.Zeller MV, Severo M, Santos AC, Drummond M. 5-Years APAP adherence in OSA patients – Do first impressions matter? Respir Med. 2013;107:2046–52. doi: 10.1016/j.rmed.2013.10.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.